Aedes aegypti mosquitoes transmit vector borne diseases yellow fever, dengue fever, Zika fever and chikungunya. Increase in global temperature is associated with expansion geographical location of this vector, resulting in more cases. Approximately 390 million DENV-infections caused by Dengue virus (DENV) occur yearly, of which 25% are symptomatic. DENV infection is self-limiting, characterised by an acute febrile stage. However, severe DENV infection can cause complications such as haemorrhagic fever in <1% of symptomatic individuals. Currently, there is no anti-viral treatment for DENV infection, and the only “efficacious” vaccine works best in DENV-exposed individuals. However, previous DENV infection can cause worse secondary “disease” if infected with a different DENV viral “strain”. Understanding T cell kinetics associated with natural DENV infection is essential to the development of better DENV vaccines.
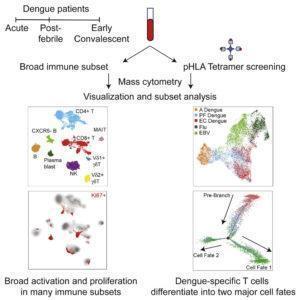
A study by Chng et al., used CYTOF and Luminex to determine cellular activation, phenotypic, memory and functional kinetics associated with acute (febrile), post-febrile and early convalescent DENV infection. As expected they showed that acute infection was associated with high levels of activation (defined by expression of Ki-67, CD38 and/or HLA-DR) by not only T cells but by NK and B cells as well. Higher levels of activation were associated with higher levels of pro-inflammatory cytokines IL-18, IL-12 and IFN-γ, and chemokines IP-10, MCP-1 and MCP-2. They showed that this pro-inflammatory milieu was responsible for non-specific activation and increased functional activity of MAITs and γδ T cells. This pro-inflammatory micro-environment was resolved once DENV infection cleared.
In addition to this, they showed that clearance of DENV infection was associated with high levels of CD57+CD127- DENV-specific CD8 T cells. These cells produce high levels of IFN-γ and cytotoxic molecules. Additionally, infection also induced a sizeable population of DENV-specific CD57-CD127+ CD8 T cells, cells associated with long-term memory. These results indicate natural DENV infection induces both long-term memory cells, as well as long-lived cells that have effector and phenotypic traits similar to terminally differentiated cells. These finding are very interesting and warrants further investigation. Results suggest that an effective DENV-vaccine should induce both cell types, as they may be the key to long-term immunity. Where effector CD57+CD127- cells can readily provide anti-viral restrictive immunity, while CD57-CD127+ generate new effector cells to provide more T cell-mediated anti-DENV immunity.
Journal Article: Chng et al., 2019. Large-Scale HLA Tetramer Tracking of T Cells during Dengue Infection Reveals Broad Acute Activation and Differentiation into Two Memory Cell Fates. Immunity.