Researchers have uncovered a key role for the complement system in driving inflammation that leads to preterm birth and fetal brain injury (Figure 1). The findings highlight complement as a potential therapeutic target to prevent preterm delivery, the leading cause of newborn complications and mortality worldwide.
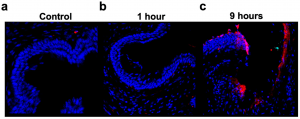
Figure 1: Deposition of complement (C3) in murine cervical tissue after LPS administration. Representative 40× immunofluorescent imaging of C3, captured at different timepoints following LPS administration; no quantitative analysis performed. (a) Naïve cervical tissue showing minimal C3 deposition. (b) Cervical tissue collected 1 h after LPS administration showing minimal, but increased C3 deposition compared to naive control tissue. (c) Cervical tissue collected 9 h after LPS administration showing increased C3 deposition compared to both naive control and 1 h post-LPS administration tissue.
Preterm birth, defined as delivery before 37 weeks and is linked to conditions such as brain haemorrhage, cerebral palsy, sepsis, and neonatal death. Current treatments focus largely on slowing labour once it begins, rather than preventing its root causes.
The team used a mouse model of infection-driven inflammation in pregnancy to test whether complement activation fuels cervical weakening and premature delivery. They found that complement activity surged within hours of infection, recruiting leukocytes to the cervix and triggering a cascade of inflammatory cytokines. This immune-driven damage compromised cervical integrity, leading to early birth.
Strikingly, treatment with a complement inhibitor prevented these changes: inflammation in both the uterus and fetal brain was reduced, leukocyte infiltration diminished, and pregnancies were prolonged, resulting in more viable offspring.
The study builds on the broader therapeutic interest in complement inhibitors, several of which are already in clinical trials for other diseases. Although none are currently approved for preventing preterm birth, this research lays the groundwork for exploring maternal complement blockade as a dual-protective therapy for mother and child.
Journal article: McElwee, E. R., et al. 2025. Complement modulation mitigates inflammation-mediated preterm birth and fetal neural inflammation. Cells.
Summary by Stefan Botha










