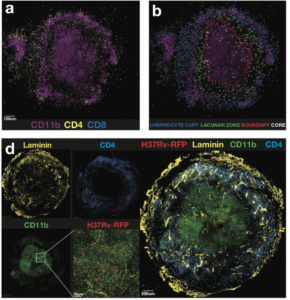
Kauffman et al., 2017: Figure 6(a) Example image showing the location of CD4þ and CD8þ cells in relation to CD11b-expressing cells. (b) Four different regions of the granuloma were identified as described in the text and CD4 T cells in each region highlighted in a different color: lymphocyte cuff (blue), lacunar zone between the lymphocyte cuff, and the macrophage core (green), boundary of the macrophage-rich core (red), and the macrophage-rich core (white). (d) Individual staining of laminin, CD11b, CD4, and RFP-Mtb on the left with a merged tile on the right.
Murine models of Mycobacterium tuberculosis (M.tb) have been instrumental in demonstrating the importance of CD4 T cells in the control of M.tb infection. Based on murine studies of M.tb infections, researchers hypothesise that T cell differentiation is a major determinant of the protective capacity of CD4 T cells. Where less differentiated M.tb-specific cells that express CXCR3 and PD-1 have superior “protective” capacity and ability to home to the site of infection, compared to more differentiated KLRG1 and CX3CR1 expressing CD4 T cells.
However, murine models of M.tb infection do have limitations, such as the inability to adequately study the granuloma because mice do not form human-like granulomas. As a result, examining intralesional leukocyte trafficking within the granuloma in murine models is not ideal. Non-human primate (NHP) models of M.tb infection recapitulate many aspects of human tuberculosis (TB) disease progression and pathology. This makes them ideal models for studying TB granulomas compared to murine studies. Researchers from the National Institutes of Health (United States of America) aimed to determine if “protective” M.tb specific responses in rhesus macaques have similar characteristics as those observed in the murine model.
Kauffmann et al., demonstrated that M.tb-specific T cells induced by infection are predominantly CXCR3+, a T cell phenotype associated with lung homing capacity. Surprisingly, little to no expression of CX3CR1 by M.tb-specific T cells was observed, regardless of anatomical location. Suggesting that unlike the murine model, ability to home to the lung is not the reason for reduced protective capacity of T cells in macaques. Further investigation of the granuloma structure suggested that a potential pitfall in T cell mediated immunity against M.tb, could be due to limited interactions between CD4 T cells and M.tb-infected macrophages.
Despite the low sample size in the Kauffmann et al. study, this research clearly highlights the need for research that determine mechanisms by which T cell mediated immunity in the granuloma can be improved. As well demonstrating the utility of NHP models over murine models, in spite of the increased costs.
Journal Article: Kauffmann et al., 2017. Defective positioning in granulomas but not lung-homing limits CD4 T-cell interactions with Mycobacterium tuberculosis-infected macrophages in rhesus macaques. Mucosal Immunology
Article by Cheleka AM Mpande











