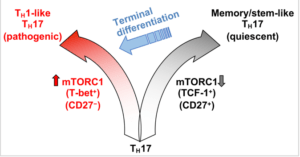
TH17 cells are functionally and metabolically heterogeneous, and are composed of a subset with stemness features but lower anabolic metabolism, and a reciprocal subset with higher metabolic activity that supports transdifferentiation into TH1 cells. These two subsets are further distinguished by selective expression of the transcription factors TCF-1 and T-bet, respectively, and discrete levels of CD27 expression. mTORC1 activation drives reprogramming of anabolic metabolism, favouring transcription that is mediated by T-bet rather than TCF-1; consequently, TH17 transdifferentiation into TH1-like TH17 cells occurs. Memory/stem-like TH17 cells can become reactivated and have the potential to undergo terminal differentiation and acquire TH1-like phenotypes. (Source Karmaus et al., 2019)
T cells are a population of lymphocytes that play a crucial role in autoimmune and pathogenic immunity. T cells are very heterogenous and exhibit high plasticity, demonstrated by their ability to undergo memory and functional differentiation depending on the pathogenic microenvironment. Numerous studies have shown that long term T cell memory is mediated by cells with stem cell-like properties such as central and stem cell memory T cells.
Helper T cells that produce IL-17 cytokine, referred to as Th17 cells, possess the ability to differentiate into IFN-γ producing Th1 cells and/or IFN-γ and IL-17 co-expressing Th1/17 cells. Additionally, Th17 cells have been shown to possess stem cell-like properties, suggesting that they also can mediate long-term memory. However, since Th17 cells are very heterogeneous, it is unclear whether all Th17 cells possess this stemness phenotype. Thus Karmaus et al., 2019 aimed to determine the heterogeneity of Th17 cells in autoimmune murine models, as well as define metabolic, functional and phenotypic characteristics associated with stemness and plasticity.
Using CD27 Karmaus et al., were able to define two distinct populations of Th17 cells, where CD27+ cells predominantly produced IL-17, while CD27- cells were more heterogenous exhibiting Th1/17 properties. CD27+ Th17 cells expressed higher levels of transcription factor T-cell factor (TCF1) than CD27- Th17 cells, a transcription factor associated with stemness (high proliferative and self-renewal capacity). Additionally, CD27- Th17 cells had higher mammalian target of rapamycin complex 1 (mTOR) signalling and Tbet expression than CD27+ Th17 cells, and were associated with higher pathology and disease progression. Using mice that were deficient in mTORC1 activity they demonstrated that mTORC1 function plays a crucial role and is required for Th17 differentiation to Th1/17 cells and down regulation of CD27.
In Summary, Karmaus et al., showed that during chronic autoimmunity Th17 cells are heterogenous, where Th17 cells with enriched stemness properties are CD27+TCF1hi, while IFN-γ expressing Th17 cells with high metabolic activity are CD27-Tbethi.
Journal Article: Karmaus et al., 2019. Metabolic heterogeneity underlies reciprocal fates of TH17 cell stemness and plasticity. Nature











