In a surprising twist to our understanding of thymic education, a recent study from the Tak Wak lab in Toronto, Canada, unveils a non-neuronal role for acetylcholine (ACh) in shaping the T cell repertoire (Figure 1). Published in Nature Immunology this work demonstrates that acetylcholine signaling through the nicotinic acetylcholine receptor subunit alpha9 (Chrna9) modulates T cell receptor (TCR) signaling thresholds, influencing both negative selection and regulatory T cell (Treg) differentiation in the thymus.
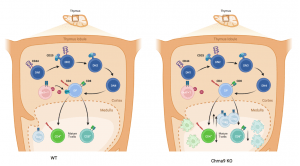
Figure 1: Acetylcholine modulates negative selection in the thymus. Acetylcholine produced by Choline Acetyltransferase (ChAT)-expressing T cells (ChAT⁺ T cells) signals through nicotinic acetylcholine receptors on double-positive (DP) thymocytes. In wild-type mice, this signaling attenuates TCR activation, thus calibrating TCR affinity and modulating negative selection. In Chrna9 knockout mice, the absence of acetylcholine receptor signaling results in unchecked TCR signaling, leading to increased negative selection, increased apoptosis, limited TCR repertoire of conventional T cells and increased thymic treg differentiation. (Created in Biorender)
Double-positive (DP) thymocytes those expressing both CD4 and CD8—transiently express Chrna9 in both human and murine thymus. To explore the functional significance of Chrna9 expression, the researchers analyzed mice deficient in this gene. Surprisingly, Chrna9 knockout (KO) mice exhibited a largely intact negative selection process and normal levels of death by neglect. However, a closer look revealed an excessive death of thymocytes via negative selection, accompanied by an unexpected increase in Treg generation. Mechanistically, the absence of Chrna9 led to heightened TCR signaling strength, suggesting that ACh acts as a brake to fine-tune the TCR signal threshold necessary for thymocyte survival.
But where is this acetylcholine coming from? The thymus lacks classical cholinergic innervation. The study elegantly shows that thymocytes themselves, particularly T cells, are the primary source of ACh in the thymus. This autocrine or paracrine cholinergic signaling modulates the sensitivity of DP cells to TCR engagement, effectively shaping the outcome of thymic selection.
Functionally, this cholinergic tuning has consequences beyond development. Chrna9 KO mice presented with an altered TCR repertoire and exhibited reduced pathology in two disease models driven by autoreactive T cells: experimental autoimmune encephalomyelitis EAE and T cell–induced colitis. These findings suggest that by modulating TCR signal strength during development, ACh may act as a key regulator of central tolerance and immune homeostasis.
This study redefines our view of neurotransmitter function, extending its influence from neurons to immune cell-intrinsic regulation. The identification of a cholinergic axis in thymic selection highlights a previously underappreciated layer of control in T cell development and opens new therapeutic avenues for modulating immune tolerance in autoimmunity and beyond.
Journal article: Liu, S, et al 2025. Cholinergic regulation of thymocyte negative selection. Nat Immunol.
Summary by Eugenio Contreras Castillo










