Since the emergence of the SARS-CoV-2 Omicron variant (B.1.1.529) in November 2021, it has become the dominant variant of concern in most countries, accounting for 99% of new infections. This is due to Omicrons high transmissibility.
Because of its 30 mutations, the Omicron variant is able to escape the majority of existing SARS-CoV-2 neutralizing antibodies (nAbs). This has led to the inability of the spike (S) protein monoclonal antibodies to neutralise Omicron. Individuals who have been previously been infected with other SARS-CoV-2 variants were reported to have minimal nAbs against Omicron, making them susceptible to reinfection.
It has been reported that a third dose or booster of available common COVID-19 vaccines is an effective measure for reducing deaths, hospitalisations, and severe disease caused by Omicron. However, Omicron is still a threat to elderly individuals, especially those with pre-existing health conditions.
In a recent study, Wu, et al., aimed to develop a messenger ribonucleic acid (mRNA) vaccine using the SARS-CoV-2 Omicron variant sequence as a target. The aim was to develop a vaccine capable of generating nAbs in multiple animal models (Figure 1 – similar results were seen in Syrian hamsters and Macaques) against the Omicron variant when compared to other commercially available vaccines.
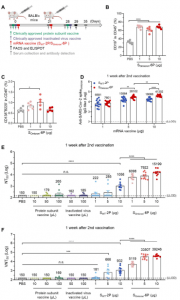
Figure 1: SOmicron-6P Induces Antigen-Specific Humoral Immune Responses in Mice. (A) Schematic diagram of immunization and sample collection schedule in mice. Female BALB/c mice were immunized on a two-dose schedule with SWT-2P, SOmicron6P, protein subunit vaccine using a dimeric form of the receptor-binding domain of wild-type SARS-CoV-2, or inactivated vaccine of wild-type SARS-CoV-2. (B-C) Percentages of (B) B cells and (C) plasma cells in spleen after immunized with different doses of SOmicron-6P. (D) The Omicron SARS-CoV-2 variant specific IgG antibody titers were determined by ELISA (lower limit of detection (LLOD) = 100). (E) Neutralization titers (NT50) were determined by recombinant vesicular stomatitis 127 virus (VSV)-based pseudovirus (Omicron variant) neutralization assay (LLOD = 150). (F) SARS-CoV-2 Omicron 50% virus-neutralization titers (VNT50) were determined by a plaque reduction neutralization test (LLOD = 150). Data are shown as mean ± SEM. Significance was calculated using one-way ANOVA with multiple comparisons tests (n.s., not significant, *p < 0.05, **p < 0.01, ***p < 132 0.001, ****p < 0.0001) (Wu, et al., 2022).
In the present study, the researchers designed and synthesised mRNA based off of the original SARS-CoV-2 strain and the Omicron variant. The mRNAs were then encapsulated in lipid nanoparticles (LNPs) in order to produce the vaccine.
The study presented the development of a Omicron-specific vaccine which provided protection in naïve animals, improving on existing mRNA vaccines with boosters.
NB to note: bioRxiv is a preprint server which publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, or guide clinical practice or treated as established information.
Journal article: Wu, Y., et al. 2022. Omicron-specific mRNA vaccine elicits potent immune responses in mice, hamsters, and nonhuman primates. bioRxiv.
Summary Stefan Botha










