Lymph nodes are central hubs of immune activity. They filter lymphatic fluid, coordinate immune cell communication, and help launch targeted responses to infection and disease. Accurately modeling this environment in vitro opens the door to several advantages: reduced reliance on animal models, greater biological relevance, personalized medicine potential.
Researchers have developed a sophisticated laboratory model that mimics the structural and functional environment of a human lymph node (Figure 1). This bioengineered “lymph node-on-a-chip” is designed to replicate key features of the immune system’s architecture—most notably, dynamic fluid flow and complex cellular interactions.
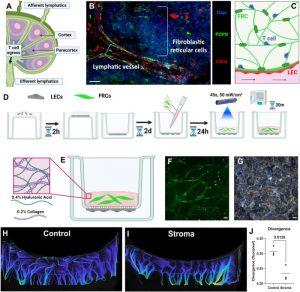
Figure 1: Lymph node stroma model development. We sought to develop a model of immune cell egress from the lymph node (a). A representative immunofluorescence image of a portion of a normal human lymph node is shown. In human lymph nodes, lymphatics (PDPN+, CD31+, visualized in red and green) and fibroblastic reticular cells (PDPN+, in green) form the structure of the T cell zone (b). Schematic of an intended structure of the T cell zone model (c). LECs were seeded on the underside of a tissue culture inset. PhotoHA-collagen gels laden with FRCs are crosslinked above the LECs and then incubated for 30 min (d). After thermal cross-linking, the final gel consists of hyaluronic acid and collagen (e). With this methodology, FRCs formed networks (f) and LECs formed an intact monolayer (g). Scale bars are 50 μm. Magnetic resonance imaging (MRI) demonstrates altered fluid transport in the presence of LN stroma (h), (i). Divergence of fluid was significantly decreased in the presence of LN stroma (j). Each data point represents a biological replicate (n = 3). Significance was determined by Students’ t-test, with significant p values (<0.05) reported on the graph.
The study outlines how this new model can serve as a powerful platform for studying immune responses, disease progression, and therapeutic interventions—all outside the human body.
While lymph nodes are best known for their role in immune surveillance, they are also common sites for cancer metastasis. The model has promising applications for studying how tumour cells spread and evade immune responses.
A unique strength of the model is its ability to simulate different physiological states, including inflammation. Inflammatory conditions increase fluid movement through lymph nodes and can trap immune cells in ways that influence their behaviour.
The researchers engineered two versions of the system: one mimicking normal conditions and another representing an inflamed environment. They found that immune cells—particularly T cells—behaved differently under inflammatory flow conditions, highlighting how tissue context can dramatically affect immune function.
This effort is part of a broader push to develop advanced tissue models that can eventually replace traditional animal experiments. By building more physiologically accurate models, researchers can better understand disease mechanisms and improve the way drugs are tested before reaching clinical trials.
Journal article: J. H. Hammel et al, 2025. Interstitial fluid flow in an engineered human lymph node stroma model modulates T cell egress and stromal change, APL Bioengineering.
Summary by Stefan Botha










