New research challenges the long-standing idea that vaginal health can be defined simply by the presence of “good” Lactobacillus bacteria or “bad” Gardnerella. The study reveals a much more nuanced picture of the vaginal microbiome (Figure 1).
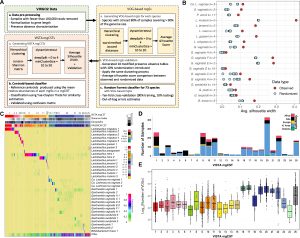
Figure 1: Development, composition, and prevalence of VISTA mgCSTs. (A) Flow diagram for construction of VISTA mgCSTs. (B) Comparison of VOG-based mgSs clusters generated from the observed and randomized data for selected species. (C) MgCST composition and basis of classification. It is important to note that “Ca. Lachnocurva vaginae” is referred to as UBA629 sp005465875 in VIRGO2, but as its genome has recently been completed, VISTA uses the newly assigned name. (D) Distribution and prevalence of mgCSTs by region where source data have originated. (E) The log10-transformed number of VOGs per sample, stratified by mgCST.
By analysing microbial communities at high resolution, researchers identified 25 distinct vaginal microbiome types. Surprisingly, six of these were dominated by Gardnerella, yet they differed significantly in their biological functions and inflammatory profiles. One Gardnerella-dominated type even resembled Lactobacillus-dominant communities in terms of its functional behaviour, highlighting that bacteria of the same species can act very differently depending on their genetic makeup.
To enable this deeper analysis, the team developed two open-source tools. VIRGO2 is a comprehensive gene catalogue containing approximately 1.7 million genes from vaginal microbes collected across multiple continents. VISTA complements this resource by classifying microbiomes based on strain-level functional characteristics rather than species alone.
The findings suggest that identifying which bacteria are present is not sufficient; understanding what they can do may be more important. This work lays the groundwork for more precise diagnostics and risk assessment in women’s health, moving beyond simplified “optimal” versus “non-optimal” classifications.
While clinical practice will not change immediately, the study provides a foundation for future research aimed at developing more personalized approaches to gynaecological care.
Journal article: Williams, A., et al. 2026. Not all vaginal microbiomes are equal: functional context shapes immune landscapes. mBio.
Summary by Stefan Botha










