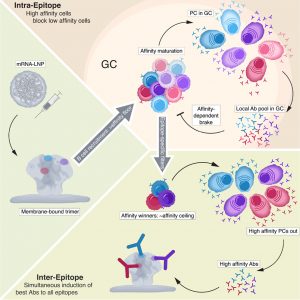
New research reveals how antibodies generated early in an immune response can influence which B cells continue to mature in germinal centres, the sites where high-affinity antibodies are refined (Figure 1).
Using an mRNA-based immunogen displaying three conserved HIV-1 envelope epitopes, researchers tracked B cells engineered to recognize specific targets with defined binding strengths. They found that B cells with higher-affinity receptors did not necessarily dominate the response for long. Instead, these high-affinity cells exited germinal centres more quickly than lower-affinity competitors.
Importantly, competition occurred within individual epitopes. When multiple B cell clones targeted the same epitope, higher-affinity clones suppressed the expansion of lower-affinity counterparts. However, this dominance was tempered by a local feedback mechanism: antibodies produced by nearby plasma cells accumulated in lymph nodes and fed back into germinal centres. This local IgG limited further affinity escalation within a given epitope, effectively setting both lower and upper thresholds for selection.
The study suggests that early, locally produced antibodies regulate germinal centre dynamics by preventing runaway affinity maturation against a single epitope. This self-limiting mechanism may promote broader immune responses by redirecting B cell selection toward alternative epitopes, a process known as epitope spreading.
These findings provide new insight into how antibody feedback shapes vaccine responses and could inform the design of next-generation mRNA vaccines aimed at eliciting broad, durable immunity.
Journal article: Yan, Y., et al. 2026. Local antibody feedback enforces a checkpoint on affinity maturation in the germinal center and promotes epitope spreading. Immunity.
Summary by Stefan Botha