Immune checkpoint inhibitors have transformed cancer treatment, but many patients, including up to 40% with melanoma, do not respond because tumors create highly immunosuppressive environments. Researchers have now developed an inhalable nanotherapy that may overcome this resistance, particularly in cancers that spread to the lungs (Figure 1).
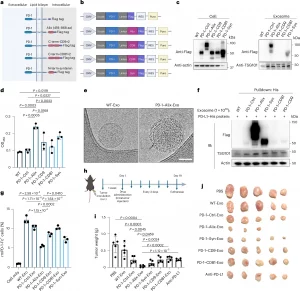
Figure 1: Systematic screening of multiple endogenous exosome display strategies. a, Schematic showing the design of the endogenous exosome display platform; aa, amino acids. b, Schematic showing the design of the construct plasmids. c, Immunoblot analysis of Flag-tagged exosomes and parental cells. Samples were loaded based on equal protein mass. Actin was used as the loading control for cells, whereas TSG101 served as the loading control for exosomes. Duplicate assays were performed with similar results; Ctrl, control. d, PD-1 expression in exosomes (n = 3 independent experiments); OD450, optical density at 450 nm. e, Cryo-electron microscopy imaging of WT-Exo and PD-1–Alix-Exo. The black arrow indicates the PD-1 corona; scale bar, 50 nm. Triplicate assays were performed with similar results. f, Immunoblotting of the pulldown assay. Duplicate assays were performed with similar results; IB, immunoblot. g, Flow cytometry showing the percentage of recombinant mouse PD-1-Fc+ tumor cells after exosome treatment (n = 3 independent experiments). See Supplementary Fig. 17 for gating strategies. h, Schematic illustrating the assessment of antitumor efficacy following treatment. Intratumoral injections of exosomes and anti-PD-L1 (at a molecular amount equivalent to PD-1 in PD-1–Alix-Exo) were initiated 1 week after tumor inoculation and administered every 3 days for a total of 19 days; s.c., subcutaneous. i, Tumor weights 19 days after treatment. j, Tumor morphologies 19 days after treatment; n = 5 independent mice for each group in h–j. P values were determined by one-way analysis of variance (ANOVA) with a post hoc Tukey’s multiple comparisons test (d and g) and two-tailed Student’s t-test (i) using GraphPad PRISM software. Exact P values are indicated. Results are presented as means ± s.d. The schematics in a and b and mouse cartoon were created with BioRender.com.
The new approach, called BEAT (Bispecific Exosome Activator of T Cells), uses naturally occurring nanoparticles known as exosomes to deliver two immune-activating proteins directly to lung tumours. One protein blocks the PD-1/PD-L1 checkpoint pathway, while the other inhibits Wnt/β-catenin signalling, a key driver of immune cell exclusion from tumours. By targeting both mechanisms at once, BEAT tackles two major barriers to effective immunotherapy.
Delivering the therapy by inhalation allows the treatment to concentrate in the lungs, the most common site of melanoma metastasis, while limiting exposure to the rest of the body. In mouse models of metastatic melanoma that were resistant to checkpoint inhibitors, inhaled BEAT led to stronger immune cell infiltration, greater tumour suppression, and fewer side effects than systemically delivered antibody therapies.
The study builds on more than a decade of work developing exosomes as safe and biocompatible drug carriers.
Journal article: Liu, S., et al. 2026. Engineering bispecific exosome activators of T cells to target immune checkpoint inhibitor-resistant metastatic melanoma. Nature Biotechnology.
Summary by Stefan Botha










