New studies show that preserving lymph nodes may be critical for effective anti-tumour immune responses (Figure 1).
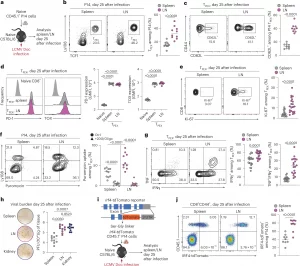
Figure 1: LNs preserve the stemness, proliferative fitness and polyfunctionality of T cells. a–g, Naive congenically marked (CD45.1+) P14 CD8+ T cells were adoptively transferred into naive wild-type (WT) (CD45.2+) mice, which were subsequently chronically infected with LCMV Doc. On day 25 after infection, CD8+ T cells in the spleen and pooled inguinal, brachial, axillary, cervical and mandibular LNs were analyzed using flow cytometry. a, Schematic of the experimental setup. b,c, Flow cytometry plots and quantification showing the frequencies of TPEX cells among P14 cells (n = 10) (b) and CD62L+ cells among TPEX cells (n = 9) (c). d, Representative histograms and quantification showing the expression of PD-1 and TOX in P14 TPEX cells in the spleen and LNs (n = 10). e, Flow cytometry plots and quantification showing Ki-67 expression in P14 TPEX cells in the spleen and LNs (n = 21). f, Flow cytometry plots and quantification showing puromycin incorporation in P14 cells in the spleen and LNs (n = 13). g, Flow cytometry plots and quantification showing IFNγ and TNF production in P14 TPEX cells from the spleen and LNs after stimulation with gp33 peptide (n = 19). h, Representative plaque assays and quantification showing viral titers in the spleen, LNs and kidney on day 25 after infection (n = 10). i,j, Naive congenically marked (CD45.1+) P14 CD8+ T cells expressing an IRF4-tdTomato fusion protein were adoptively transferred into naive WT (CD45.2+) mice, which were subsequently infected with LCMV Doc. On day 25 after infection, CD8+ T cells in the spleen and pooled inguinal, brachial, axillary, cervical and mandibular LNs were analyzed using flow cytometry. i, Schematic of the Irf4-tdTomato locus and experimental setup. j, Representative flow cytometry plots and quantification showing the expression of IRF4 in P14 cells in the spleen and LNs (n = 10). Flow cytometry plots are representative. b–h,j, The dots represent individual mice; the bars represent the median. Quantification and statistics were done using an unpaired two-tailed t-test (b,c,e,g,j) and a one-way analysis of variance (ANOVA) (d,f,h) and are based on all data points across at least two independent experiments. GMFI, geometric mean fold increase.
Lymph nodes are often removed during cancer surgery to prevent tumour spread. However, new research suggests this long-standing practice may unintentionally weaken the body’s ability to fight cancer, particularly when patients receive immunotherapy.
Across two complementary studies published, researchers demonstrate that lymph nodes are not passive immune waystations. Instead, they act as essential training hubs that sustain a special population of immune cells required for durable anti-cancer immunity.
The studies focused on a subset of CD8⁺ T cells often described as stem-like T cells. These cells are crucial because they:
- Persist long-term during chronic disease
- Self-renew
- Generate waves of short-lived “killer” T cells that directly attack tumours or infected cells
The researchers found that lymph nodes provide a unique molecular environment that allows these stem-like T cells to survive, proliferate, and differentiate effectively. In contrast, other immune organs such as the spleen could not support this process to the same extent.
As a result, lymph nodes act as a renewable source of tumour-killing T cells, especially during chronic infection and cancer, conditions where sustained immune pressure is essential.
Modern immunotherapies, including immune checkpoint inhibitors and CAR T-cell therapies, rely on the immune system’s ability to continuously generate effective T cells. The new findings show that:
- Stem-like T cells originating in lymph nodes are the primary drivers of successful immunotherapy
- T cells residing within tumours themselves are far less capable of sustaining long-term anti-tumour responses
- Removing lymph nodes can reduce the immune system’s capacity to respond, even when powerful immunotherapies are used
This helps explain why some patients respond exceptionally well to immunotherapy, while others do not, the health and availability of lymph nodes may be a determining factor.
One of the studies identified specific molecular programs, including KLF2-dependent signalling pathways, that are active in lymph nodes and enable stem-like T cells to mature into effective cancer-killing cells. These signals are much weaker or absent in other tissues.
Understanding these pathways opens the door to:
- Designing therapies that protect or enhance lymph node function
- Developing immunotherapies that better exploit lymph-node-derived immune cells
- Improving patient stratification based on immune architecture, not just tumour features
The findings suggest a shift in how clinicians and researchers think about cancer treatment:
- Surgical strategies may need to balance cancer control with immune preservation
- Immunotherapies may be more effective when lymph node integrity is maintained
- Future treatments could deliberately target lymph nodes to amplify anti-tumour immunity
While the work was conducted in preclinical models, clinical studies are already underway to test whether these mechanisms hold true in patients receiving immunotherapy.
Together, these studies reinforce a growing idea in immuno-oncology: successful cancer treatment depends not only on targeting tumours, but also on protecting the immune ecosystems that sustain long-term defence.
Lymph nodes, once viewed mainly as sites of potential cancer spread, are now emerging as critical allies in the fight against cancer.
Journal articles:
Tsui C, Heyden L, et al. 2025. Lymph nodes fuel KLF2-dependent effector CD8+ T cell differentiation during chronic infection and checkpoint blockade. Nature Immunology.
Wijesinghe SKM, Rausch L, et al. 2025. Lymph-node-derived stem-like but not tumor-tissue-resident CD8+ T cells fuel anticancer immunity. Nature Immunology.
Summary by Stefan Botha










