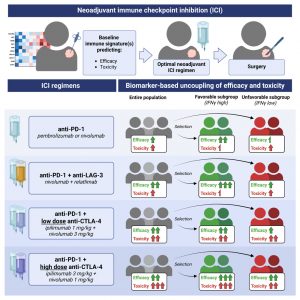
New analysis suggests immune signatures can guide safer, more effective checkpoint inhibitor combinations in melanoma (Figure 1).
Immune checkpoint inhibitors (ICIs) have transformed melanoma treatment, but not all patients benefit and many experience serious immune-related adverse events (irAEs). A long-standing challenge in the field has been balancing efficacy against toxicity, particularly when combining agents such as anti–PD-1 and anti–CTLA-4 therapies.
A new report suggests this balance may be achievable through immune-guided personalization. By analysing how pathological response and toxicity relate to baseline immune activity, the researchers show that the same treatment can behave very differently depending on a patient’s immune state.
The team conducted a meta-analysis of neoadjuvant melanoma trials, comparing four immune checkpoint regimens:
- Anti–PD-1 alone
- Anti–PD-1 + low-dose anti–CTLA-4
- Anti–PD-1 + high-dose anti–CTLA-4
- Anti–PD-1 + anti–LAG-3
As expected, increasing CTLA-4 intensity generally increased both toxicity and response rates at the population level. But this broad view masked a crucial insight.
When patients were stratified by their baseline interferon-gamma (IFN-γ) gene signature, a marker of pre-existing immune activation, a divergence emerged.
- In IFN-γ–high tumours, adding (especially high-dose) anti–CTLA-4 substantially increased toxicity but did not improve efficacy over anti–PD-1 alone.
- In contrast, in IFN-γ–low tumours, adding high-dose anti–CTLA-4 significantly improved response rates, while toxicity remained comparatively low.
In other words, patients with “cold” tumours benefited from immune escalation, while those with “hot” tumours paid the price in toxicity without gaining additional benefit.
These findings suggest that risk of non-response and risk of toxicity are not the same and can be disentangled using immune biomarkers.
Rather than treating all patients with the same escalation strategy, baseline immune profiling could help answer two critical questions:
- Does this patient need stronger immune activation to respond?
- Is this patient already primed and therefore more vulnerable to immune toxicity?
The study supports a shift away from one-size-fits-all checkpoint combinations and toward adaptive treatment strategies, where therapy is escalated or de-escalated based on immune context rather than trial averages.
Such an approach could:
- Improve outcomes in patients with immune-cold tumours
- Reduce unnecessary toxicity in immune-hot patients
- Enable more rational sequencing of PD-1, CTLA-4, and emerging checkpoints like LAG-3
Baseline immune signatures, particularly IFN-γ activity, may identify which melanoma patients need intensified checkpoint therapy and which should avoid it. This work moves immunotherapy closer to a future where efficacy and safety can be optimized independently, guided by each patient’s immune biology.
Journal report: Lucas, M.W., et al. 2025. Immune signature-based uncoupling of checkpoint inhibitor efficacy and toxicity. Immunity.
Summary by Stefan Botha