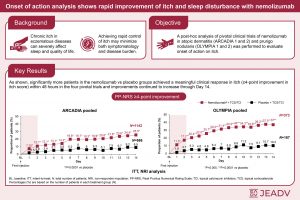
Clinical trial data show nemolizumab eases itch and improves sleep within days for people with severe eczema and prurigo nodularis (Figure 1).
For people living with eczema, relentless itching can be more than uncomfortable it can disrupt sleep, impair daily functioning, and significantly reduce quality of life. Now, new research suggests that a recently approved injectable drug may offer rapid relief, sometimes within just 48 hours.
A follow-up analysis of large clinical trials found that nemolizumab (Nemluvio) relieved itch far more quickly than placebo and helped patients sleep better within days.
Nemolizumab is a monoclonal antibody that targets a receptor involved in activating immune signals that drive itch and inflammation in skin diseases. By blocking this pathway, the drug interrupts one of the core mechanisms underlying eczema-related itching.
In 2024, the U.S. Food and Drug Administration (FDA) approved nemolizumab for adults with moderate-to-severe atopic dermatitis (eczema) and prurigo nodularis, a condition marked by intensely itchy skin nodules that often coexist with eczema.
Researchers re-examined data from the pivotal clinical trials that supported FDA approval, covering nearly 2,300 patients. Their focus was on how quickly patients experienced relief.
The results were:
- Within 48 hours, itch improved in nearly 11% of eczema patients receiving nemolizumab, compared with 3% on placebo
- Among patients with prurigo nodularis, 17% experienced rapid itch relief, versus ~4% with placebo
- Sleep quality improved within two days for 10% of eczema patients and over 13% of prurigo nodularis patients, roughly double to triple the rates seen with placebo
By two weeks, the benefits became even clearer:
- Around 25% of eczema patients
- More than one-third of prurigo nodularis patients
showed clinically meaningful improvements in both itch severity and sleep. Reducing itch not only eases discomfort but can also break the cycle of scratching, skin damage, and sleeplessness that fuels disease severity.
For clinicians, the findings reinforce nemolizumab’s role as a fast-acting targeted therapy, especially valuable for patients whose symptoms are severe or poorly controlled with existing treatments.
Eczema is driven by immune dysregulation that weakens the skin barrier and amplifies inflammatory signalling. By precisely targeting itch-related immune pathways, nemolizumab represents a broader shift toward mechanism-based, patient-centered therapies, focused not just on reducing inflammation, but on improving day-to-day life.
Nemolizumab provides rapid itch relief, often within two days, and improves sleep in patients with eczema and prurigo nodularis. These findings highlight its potential to quickly ease one of the most burdensome symptoms of chronic inflammatory skin disease.
Journal article: Ständer, S., et al. 2025. Rapid improvement of itch with nemolizumab in atopic dermatitis and prurigo nodularis phase 3 studies, Journal of the European Academy of Dermatology and Venereology.
Summary by Stefan Botha