Scientists develop a non-invasive nanomedicine that delivers immune-stimulating therapy directly to brain tumours (Figure 1).
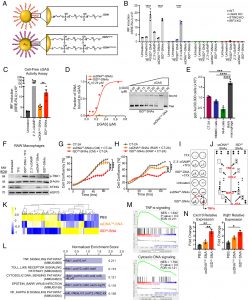
Figure 1: cGAS-agonistic ISD45-SNAs activate the cGAS-STING pathway in murine macrophages. (A) Schematic of the control ssDNA- and cGAS-agonistic ISD45 oligonucleotide-carrying SNA architectures. (B) IRF3 reporter assay in wild-type (WT), cGAS, STING, IRF3 knockout (KO) RAW-LuciaTM macrophages treated with indicated oligonucleotides in linear form or in SNA format, TFX- transfection reagent (C) IRF3 reporter assay in cGAS-KO RAW-LuciaTM macrophages expressing Lucia luciferase under the control of an ISG54 minimal promoter. Cells were transfected with reaction mixtures obtained from cell-free cGAS activation assays to measure IRF3 induction. (D) Electrophoretic mobility shift assay (EMSA) of ssDNA45- and ISD45-SNAs and recombinant human cGAS protein. The picture of the gel (Right panel) shows the fraction of bound and free SNAs as a function of increasing cGAS protein concentrations. The dissociation constant (Kd) was determined using the Hill–Langmuir equation. (E) Inductively coupled plasma mass spectrometry (ICP-MS) analysis for gold quantification in SNA-treated tumor cells (CT-2A), Human Brain Microvascular Endothelial Cells (HBMECs), Normal human astrocytes (NHAs), and RAW LuciaTM macrophages measured as part per billion (ppb) gold (Au)/30,000 cells. (F) Immunoblot analysis of phospho-TBK1 and STING in RAW LuciaTM macrophages. β-actin and Hsp70 are shown as the respective loading controls. Molecular weights (MWs) of the indicated proteins are shown on the Left. TFX, transfection agent. (G and H) Confluency of CT-2A cells exposed to conditioned media (CM) harvested from RAW LuciaTM macrophages treated with indicated SNAs and or, as shown in (H) cocultured with RAW LuciaTM macrophages treated with indicated SNAs. (I and J) Assessment of cytokine production of RAW LuciaTM macrophages in response to treatment with linear and SNA-complexed oligonucleotides using cytokine arrays. (K) Differential Expression Gene Clustering Heatmap for RAW LuciaTM macrophages treated with PBS, ssDNAT45-SNA, and ISD45-SNAs. (L) Top 5 Kyoto Encyclopedia of Genes and Genomes-enriched pathways identified by gene set enrichment analysis (GSEA), with a false discovery rate (FDR) < 25% of the t statistic, sorted by normalized enrichment scores (NES). FDR-corrected P-values are displayed to the Right of each bar. (M) GSEA plots of TNF-α and cytosolic DNA signaling pathways. Green curves indicate the enrichment profile. The NES, P-value, and FDR (cutoff value < 25%) are indicated within each graph. (N) RT-qPCR analysis of fold change of Cxcl10 and Ifnb1 mRNA expression in RAW LuciaTM macrophages. Shown is the mean ± SEM. *P < 0.05; **P < 0.001; ****P < 0.00001, determined by one-way ANOVA with Tukey’s multiple comparisons test.
Glioblastoma, the most common and aggressive brain cancer, has long resisted effective treatment. Now, researchers have created a groundbreaking, non-invasive nanotherapy that activates the brain’s immune system to fight the disease, using nothing more than nasal drops.
Their new approach uses spherical nucleic acids (SNAs), tiny gold nanoparticles densely coated with short strands of DNA, to carry immune-activating molecules straight into the brain through the nasal passages. Once there, the nanostructures stimulate powerful immune pathways that help the body recognize and attack tumour cells.
Glioblastoma is notoriously difficult to treat because of its location and because it’s an immunologically “cold” tumour, one that hides from immune surveillance. The team aimed to awaken the brain’s immune defences by activating a cellular alarm system called the STING (stimulator of interferon genes) pathway, which triggers inflammation when cells detect foreign DNA.
Earlier attempts to use STING-activating drugs required direct injections into brain tumours, an invasive and impractical procedure for patients already in fragile health. By contrast, the new SNA nanodrops reach the brain noninvasively through the nasal cavity, travel along the nerve connecting the nose and brain, and deliver their therapeutic cargo precisely to immune cells within the tumour.
Once inside the brain, the nanomedicine triggered immune cells to switch on the STING pathway, unleashing an inflammatory response inside the tumour microenvironment. When combined with drugs that activate T cells, this two-dose treatment not only eradicated brain tumours in mice but also prevented recurrence, suggesting long-term immune memory.
Importantly, the treatment was highly targeted: immune activation occurred only in the tumour and nearby lymph nodes, minimizing potential side effects elsewhere in the body.
Researchers have developed nasal “nanodrops” that carry immune-stimulating molecules directly to brain tumours, eliminating glioblastoma in mice and preventing relapse. This non-invasive nanomedicine could one day offer patients a powerful, safer alternative to invasive treatments, turning a deadly brain cancer into a disease the immune system can finally fight.
Mahajan, A.S. et al., 2025. cGAS-agonistic spherical nucleic acids reprogram the glioblastoma immune microenvironment and promote antitumor immunity. Proceedings of the National Academy of Sciences.
Summary by Stefan Botha










