Scientists discover the molecular switch that drives tissue-resident memory T cell development, opening new doors for cancer, infection, and autoimmune therapies.
Our immune system relies on a hidden army of specialized cells that never leave their post. Known as tissue-resident memory T cells (TRM), these long-lived sentinels stay behind in organs such as the lungs, liver, and skin after an infection or tumour appears, ready to respond instantly if the threat returns.
Researchers have identified a key molecular signal that helps T cells become these highly protective TRM cells (Figure 1). The discovery reveals how a cell-surface receptor called GPR25 acts as a critical driver of TRM cell formation and could be a promising new drug target to enhance immune defence.
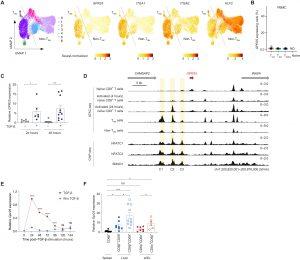
Figure 1: TGF-β induces expression of GPR25 in CD8 T cells. (A). Single-cell transcriptomes of human lung CD8 T cells from the DICE database (46), displayed using uniform manifold approximation and projection (UMAP). Seurat-normalized expression levels of the indicated transcripts in single cells are shown. The diagonal line represents the separation between TRM and non-TRM cells. (B) Bar plots showing the percentage of cells expressing GPR25 in circulating CD8 T cell subsets from the DICE database (46). (C) RT-qPCR analysis of GPR25 expression levels in naïve human CD8 T cells stimulated with anti-CD3 and anti-CD28 in the presence or absence of TGF-β for 24 and 48 hours. (D) UCSC genome browser tracks for genes in the extended GPR25 locus (50 kb), ATAC-seq tracks in the indicated CD8 T cell populations (top) and ENCODE chromatin immunoprecipitation sequencing tracks for NFATC1, NFATC3, and SMAD1 (bottom). C1, C2, and C3 cis-regulatory regions are highlighted. (E) Gpr25 expression levels in naïve murine CD8 T (CD8+CD44loCD62Lhi) stimulated with anti-CD3 and anti-CD28 in the presence or absence of TGF-β at the indicated time points. (F) Gpr25 expression levels in CD8β+ T cells (spleen), CD8β+CD69+ and CD8β+CD69− T cells (liver), and CD8α+CD8α + and CD8α+CD8β+ T cells (siIELs) isolated from unmanipulated WT C57BL/6J mice (>90 days old). Graphs in (B) depict median and interquartile range for each of the 18 donors across cell types. Graphs in (C) and (F) depict means ± SEM. Graphs in (E) depict means ± SD. (C), (E), and (F) are representative of two independent experiments (n = 5 to 11). Statistical significance was determined by two-tailed, unequal variance Student’s t test. ns, no significance.
Unlike circulating T cells that patrol the bloodstream, TRM cells stay embedded in tissues, forming a first line of defence against viruses, cancers, and other local threats. The new study reveals that TRM development hinges on a signalling cascade controlled by GPR25, a receptor found at unusually high levels on TRM cells. The researchers discovered that GPR25 is activated by the cytokine TGF-β, a well-known regulator of immune cell behaviour. Once engaged, GPR25 helps sustain TGF-β signalling, triggering T cell differentiation – the process by which ordinary memory T cells transform into tissue-resident specialists.
In genetically engineered mice lacking GPR25, this signalling pathway faltered. As a result, the animals failed to maintain normal TRM populations in tissues such as the lungs and liver, confirming that GPR25 is essential for building and sustaining these immune sentinels.
Excitingly, GPR25 belongs to the G protein–coupled receptor (GPCR) family, a class of molecules that already serve as the target for around 30% of all FDA-approved drugs. Because GPCRs sit on the cell surface, they are particularly accessible to therapeutics.
Researchers have pinpointed GPR25 as the molecular signal that helps build and sustain the immune system’s “resident defenders,” the tissue-resident memory T cells. The finding not only deepens understanding of immune memory but could lead to new drugs that fine-tune immune protection, boosting it in cancer and infections, or suppressing it in autoimmunity.
Journal article: Feng, H., et al. 2025. GPR25 promotes the formation of lung and liver tissue-resident memory CD8 T cells, Science Immunology.
Summary by Stefan Botha










