The tumour microenvironment (TME) is not just made up of cancer cells, it’s a dynamic ecosystem of immune cells, stromal cells, blood vessels, and signalling molecules, all constantly reshaping as a tumour grows. Recent evidence has highlighted a surprising new player within this system: microbes living inside tumours themselves (Figure 1). These microbes produce metabolites, small bioactive molecules, that can directly influence how immune cells behave in the TME and ultimately affect how well a patient responds to cancer immunotherapy.
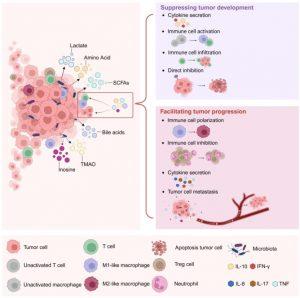
Figure 1: Metabolites from intratumoural microbes exert significant regulatory effects on the tumour microenvironment (TME) and cancer immunotherapy. Various intratumoural microbes can influence the TME through a diverse array of metabolites including lactate, amino acids, short-chain fatty acids (SCFAs), bile acids, trimethylamine N-oxide (TMAO), and inosine. These metabolites affect the TME through diverse mechanisms: 1) Lactate primarily plays a tumour-promoting role in the TME by inhibiting IFN-γ production and/or influencing the emergence of M2 macrophages and regulatory T cells (Tregs), thereby facilitating cancer progression. Notably, lactate can also significantly promote intratumoural angiogenesis. The increased intratumoural vasculature not only facilitates energy acquisition by cancer cells but also provides critical opportunities for haematogenous metastasis. 2) Amino acids, encompassing various derivatives produced through intratumoural microbial metabolism, primarily inhibit tumour development by enhancing T cell infiltration in tumour tissues and regulating cytokine secretion. It is noteworthy that certain amino acid derivatives can mediate the reduction of neutrophils in the TME, thereby exerting potent tumour growth inhibitory effects. 3) SCFAs, intratumoural microbial metabolites that predominantly inhibit tumour progression, not only regulate the production and secretion of various cytokines (IL-10, IL-17, IFN-γ) but also directly interact with cancer cell membrane surface receptors to promote cancer cell apoptosis. 4) Bile acids, as intratumoural microbial metabolites that play significant roles in the TME, exhibit complex functions. Different bile acids exhibit distinct mechanisms of action, exerting differential effects on tumour progression through the enhancement of enzyme activity, modulation of cytokine secretion, and regulation of tumour cell proliferation. 5) TMAO and inosine, two key metabolites of intratumoural microbes, influence cancer progression by modulating immune cell activity.
While most research has focused on gut microbiota and their metabolites in cancer, this review highlights the importance of distinguishing them from intratumoural microbes, which produce metabolites locally within tumours. These tumour-resident metabolites include glucose by-products like lactate, amino acids and derivatives, lipid metabolites, short-chain fatty acids, bile acids, trimethylamine N-oxide, and inosine. Once released into the TME, they can modulate cytokine secretion and immune cell activity, tipping the balance between immune suppression and immune activation.
The authors systematically map out how these metabolites interact with the TME:
- Glucose-derived metabolites like lactate often fuel immune suppression, dampening T cell and NK cell activity.
- Amino acids and their metabolites can either suppress or activate immune responses, depending on the pathway involved.
- Lipids and lipid-derived metabolites may reshape immune cell metabolism and alter their capacity to fight tumours.
- Other metabolites, such as bile acids and inosine, can act as signalling molecules with far-reaching effects on immune regulation.
Because immunotherapy depends on unleashing the body’s immune system against tumours, these microbial metabolites could strongly influence treatment outcomes. Some may blunt immunotherapy effectiveness by suppressing anti-tumour immunity, while others might boost it.
This review highlights the need for interdisciplinary integration of microbiology, oncology, and immunology to fully understand how intratumoural microbes shape cancer biology. By defining the mechanisms through which tumour-resident microbial metabolites act, researchers can identify new biomarkers for predicting immunotherapy response and even design novel treatment strategies that manipulate the intratumoural microbiome or its metabolites.
Journal article: Situ, Y., et al. 2025. The metabolic dialogue between intratumoural microbes and cancer: implications for immunotherapy. eBioMedicine.
Summary by Stefan Botha










