In a recent study, researchers have uncovered a molecular brake that limits the ability of CD8⁺ T cells to expand and fight cancer (Figure 1). The team describe how in vivo CRISPR–Cas9 screens identified STUB1, an E3 ubiquitin ligase, as a key inhibitor of antitumour T cell responses.
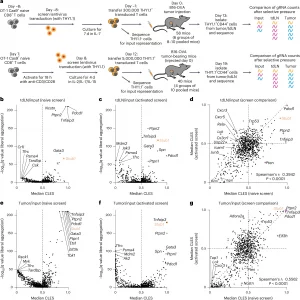
Figure 1: In vivo CRISPR screening identifies STUB1 as a negative regulator of CD8+ T cells in tumors. a, Schematic of the naive (top) and activated (bottom) CD8+ T cell-screening approaches. b–g, Screen results for the naive and activated screens for tdLN normalized to input (b–d) and tumor normalized to input (e–g). Volcano plots are shown of the median common language effect size (CLES) of the replicate pools of mice at the gRNA level and the q value for enrichment of each gene at the gRNA-UMI level in the naive T cell screen (b and e) or activated T cell screen (c and f). Comparison of the median common language effect size at the gRNA level for the naive and activated T cell screens (d and g) (naive screen: n = 6 groups of 8–10 pooled mice per group; performed twice; one-sided FAUST test on gRNA-UMI counts with liberal setting: Fisher’s combined test for all biological replicates with a Benjamini–Hochberg correction; activated screen: n = 4 groups of 10 pooled mice per group; performed once; one-sided FAUST test on gRNA-UMI counts with liberal setting and Benjamini–Hochberg correction). Spearman’s correlation was used to test the significance of correlation.
Using a melanoma mouse model, the researchers systematically disrupted nearly 900 druggable genes in CD8⁺ T cells. Among the top hits was STUB1, whose loss dramatically boosted T cell accumulation in tumours and improved tumour control. Mechanistically, STUB1 partners with the adaptor protein CHIC2 to regulate cytokine receptor stability. When either gene was deleted, cytokine receptors such as IL-27Rα and IFNγR1 were upregulated, enhancing T cell responsiveness and effector function.
This effect was not broad but selective: STUB1-CHIC2 appeared to target specific cytokine receptor families for degradation, acting as a fine-tuned regulator of signalling. Elevated IL-27Rα expression was particularly important, as it amplified CD8⁺ T cell activity against tumours.
The findings have therapeutic implications on two levels. First, tumour cells lacking STUB1 are already known to be more vulnerable to immune attack; this study now shows that inhibiting STUB1 within T cells themselves can further strengthen antitumour immunity. Together, this dual vulnerability highlights STUB1 as an especially attractive target for immunotherapy. Second, the work provides a genetic proof-of-concept that manipulating cytokine receptor turnover can enhance T cell persistence and function in hostile tumour environments.
By revealing the STUB1-CHIC2 axis as a critical checkpoint in T cell biology, this study opens the door to new therapeutic strategies aimed at re-arming the immune system against resistant cancers.
Journal article: LaFleur, M. W. et al. 2025. A STUB1–CHIC2 complex inhibits CD8+ T cells to restrain tumor immunity. Nat. Immunol.
Summary by Stefan Botha










