A rare immune condition, caused by mutations in the ISG15 gene, has revealed a remarkable biological paradox: while affected individuals are more vulnerable to certain bacterial infections, they appear resistant to virtually all viral diseases. Researchers shows how this phenomenon could be translated into a short-term, broad-spectrum antiviral therapy with potential applications in future pandemics (Figure 1).
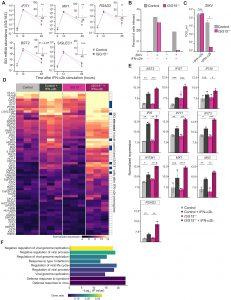
Figure 1: ISG15 deficiency inspires the identification of a nominal collection of 10 ISGs with broad-spectrum antiviral potential. (A) Relative mRNA time-course quantification of IFIT1, BST2, RSAD2, MX1, and SIGLEC1 from ISG15−/− individuals (n = 3) or control (n = 3) hTERT-immortalized fibroblasts treated with IFN-α2b (1000 IU/ml) for 12 hours, washed, and allowed to rest for 36 hours posttreatment. Not significant (n.s.); **P < 0.01, using paired, two-sided Welch’s t tests. Data are shown as mean ± SD (n = 3 biological replicates). Missing error bars indicate either very small SDs or that the lower error bars extend below zero, which could not be displayed on a logarithmic scale. (B) After the 48-hour combined IFN-α2b stimulation and resting period, cells were infected with ZIKV (PRVABC59) at an MOI of 1.0 overnight, fixed, subjected to live/dead and viral staining for ZIKV envelope protein (4G2 antibody), and processed and analyzed by flow cytometry. The percentage of infected cells was quantified. (C) Viral titers in supernatants from ZIKV-infected cells were quantified by TCID50 assay using control fibroblasts. n.s.; *P < 0.05 by two-sided paired t test with Welch’s correction. Values were log transformed before analysis, and data represent mean ± SD (n = 3 biological replicates). (D) Differentially expressed IFN-I–responsive genes between unstimulated and 12-hour IFN-α2b–stimulated, 24-hour rested fibroblasts from control and ISG15−/− individuals. Heatmap color intensity depicts variance-stabilized, normalized expression values. The row annotation bar describes overlap of elevated ISGs in both ISG15−/− and control IFN-α2b–exposed conditions (relative to unstimulated; genotype matched). (E) Normalized expression values of 10 selected ISGs elevated in both ISG15−/− and control IFN-α2b–exposed conditions, compared with respective unstimulated controls. IFI30 and MX1 did not meet the significance cutoff but were included because of their antiviral functions. IFIH1 was excluded because of its role as a double-stranded RNA sensor and signal potentiator of the IFN-I response. n.s.; *Padj < 0.05, **Padj < 0.01, ***Padj < 0.001, and ****Padj < 0.0001 by Benjamin-Hochberg method. (F) GO enrichment analysis of differentially IFN-I–responsive genes indicated in (D). Gene ratio indicates the proportion of genes in the input list that are associated with each GO term compared with the total number of genes shown in (D).
The Rare Condition: ISG15 Deficiency
- Genetic cause: Mutations impairing ISG15, an immune regulator.
- Consequence: Persistent, low-level systemic inflammation that unexpectedly biases the immune system toward antiviral defense.
- Observation: Patients exposed to viruses such as influenza, measles, mumps, and SARS-CoV-2 showed immune signatures of infection but reported no overt illness or symptoms.
The team designed an experimental therapy to reproduce this antiviral state temporarily without the long-term inflammation seen in ISG15-deficient patients.
- Approach:
- Instead of fully disabling ISG15, the therapy selectively induces 10 key proteins (out of 60 normally upregulated) that drive antiviral activity.
- Delivered using lipid nanoparticles carrying mRNA, similar to COVID-19 vaccines, into the lungs via intranasal administration.
- Host cells then produce the protective proteins, creating a transient antiviral shield.
Preclinical Results
- Models tested: Mice and hamsters.
- Outcomes:
- Prevented replication of influenza and SARS-CoV-2.
- Reduced disease severity.
- In vitro, the therapy blocked every virus tested so far.
- Duration of effect: Currently estimated at 3-4 days.
Scientific & Clinical Implications
- Demonstrates that mimicking rare immune states can inspire innovative therapies.
- Could be deployed for:
- First responders during outbreaks.
- Elderly or high-risk populations in nursing homes.
- Family members of infected individuals, regardless of the viral pathogen.
Unlike vaccines, this approach does not block adaptive immune memory, allowing the body to still develop long-term protection after natural exposure.
Challenges Ahead
- Drug delivery: Efficient, targeted uptake of the nanoparticles into lung tissue remains the biggest hurdle.
- Dosing optimization: Current protein levels may be insufficient for immediate clinical use.
- Duration of protection: Needs to be extended beyond a few days to maximize utility.
By leveraging insights from rare immune conditions, this work exemplifies how curiosity-driven research can uncover entirely new therapeutic frontiers.
Journal article: Akalu, Y. T., et al. 2025. An mRNA-based broad-spectrum antiviral inspired by ISG15 deficiency protects against viral infections in vitro and in vivo. Science Translational Medicine.
Summary by Stefan Botha










