A new study reveals that central memory CD8+ T cells are established in early childhood and persist across the human lifespan, underpinning long-term immunity to influenza (Figure 1).
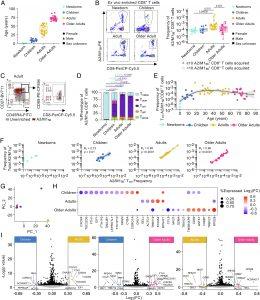
Figure 1: Central memory T cells dominate influenza-specific CD8+ T cells across the human lifespan. (A) Age of HLA-A*02:01-expressing newborns, children, adults, and older adults. (B) Representative FACS plots of enriched tetramer-specific A2/M158+CD8+ T cells and frequencies of total A2/M158+CD8+ T cells. (C) Representative FACS plots of A2/M158+CD8+ Tcm-like (CD27+CD45RA−) cells, Tem-like (CD27−CD45RA−), Temra-like (CD27-CD45RA+), Tnaive-like (CD27+CD45RA+CD95−), Tscm-like (CD27+CD45RA+CD95+) cells. Gray dots represent total CD8+ T cells in unenriched samples, red dots A2/M158+CD8+ T cells in enriched samples. Gating across age groups shown in SI Appendix, Fig. S1B. (D) Stacked bar plots of memory phenotype proportion across age groups. Statistical significance was determined with two-way ANOVA with a two-sided Tukey’s test for multiple comparisons. (E) Frequency of Tcm A2/M158+CD8+ T cells across age. (F) Correlation of the frequency of total A2/M158+CD8+ T cells and frequency of A2/M158+CD8+ Tcm-like cells using Spearman’s rank correlation (Rs) (n = 10; Newborns, n = 12; Children, n = 30; Adults, n = 22; Older Adults). (G) Principal Component Analysis of scRNASeq data of donors. Each dot represents a donor and is colored by age group. (H) Bubble plot of differentially expressed genes (DEG) from A2/M158+CD8+ Tcm from children, adults, and older adults. DEGs were identified by pairwise comparison with a two-side hurdle model (MAST) without correction for multiple comparison (P < 0.05). (I) Pairwise volcano plots of DEGs in A2/M158+CD8+ Tcm in children, adults, and older adults. Significant genes were those with |Log2(fold change)| >0.3 and P-value <0.05. (A and C) A2/M158+CD8+ Tcm frequencies of 0 are plotted as 10−7. Donors with <10 total A2/M158+CD8+ T cell counts were excluded from phenotypic analysis.
Key Findings
- Study design: First lifespan-wide analysis of influenza-specific central memory CD8+ T cells (from newborns to older adults).
- Results:
- These cells are detectable from early life and remain present into older age.
- While their numbers decline in elderly individuals, their quality and memory potential are preserved.
- TCR repertoire and gene expression analyses confirmed that the most effective influenza-specific clones are maintained in this central memory pool.
- Immunological role:
- Central memory CD8+ T cells act as an immune reservoir, rapidly generating effector T cells upon re-exposure to influenza, even when the virus mutates.
Scientific & Clinical Significance
- Demonstrates that lifelong influenza immunity relies on central memory CD8+ T cells, not just circulating effector T cells.
- Supports vaccine strategies that boost or harness central memory T cell populations for stronger, more durable protection.
- Suggests that older adults, though vulnerable to influenza, still retain a memory T cell pool that can be enhanced by next-generation vaccines.
Impact on Influenza Vaccine Design
- Current vaccines primarily target antibodies and short-lived T cell responses.
- This study highlights central memory CD8+ T cells as a prime target for universal influenza vaccine development, particularly to protect older adults and high-risk populations.
- Provides a foundation for T cell-based vaccine platforms aimed at long-term, cross-strain influenza immunity.
Central memory CD8+ T cells form a lifelong immune reserve against influenza, and targeting these cells offers a promising path toward more durable and universal flu vaccines.
Journal article: Menon T, et al. 2025. Central memory T-cells with key TCR repertoires and gene expression profiles dominate influenza CD8+ T-cell pools across human lifespan. PNAS.
Summary by Stefan Botha










