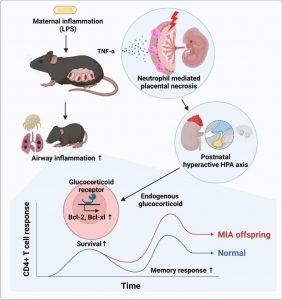
For the first time, researchers have shown that inflammation within the placenta during pregnancy can directly reprogram the fetal immune system, leading to heightened allergic responses after birth (Figure 1). The findings offer a potential pathway for early prediction and prevention of pediatric allergic diseases, including asthma.
Key Discoveries:
- Maternal inflammation, triggered experimentally in mice using lipopolysaccharide (LPS), induced placental inflammation.
- This led to increased TNF-α levels in the placenta, activating neutrophils and causing inflammatory tissue damage.
- Placental inflammation altered the offspring’s stress response regulation system, increasing glucocorticoid secretion.
- Result: Enhanced survival and memory differentiation of T cells, which persisted postnatally.
- Placental TNF-α Surge → Neutrophil activation → Placental damage
- Fetal stress axis modulation → Elevated glucocorticoids
- Memory T-cell longevity and potency → Stronger immune memory post-birth
- Postnatal allergen exposure (house dust mite):
- Strong eosinophilic inflammation in airways
- Excessive immune activation, increasing cells linked to allergy and asthma
This is the first study to link maternal placental inflammation to altered fetal immune memory leading to exaggerated allergic responses. This study provides a mechanistic bridge between prenatal inflammatory events and pediatric asthma/allergy susceptibility and opens opportunities for:
-
- Biomarker development to predict allergy risk at birth
- Preventive interventions during pregnancy
This landmark KAIST study identifies the placenta as a key mediator between maternal inflammation and the child’s lifelong immune trajectory, shaping allergic risk before birth.
Journal article: Kwon, M. S., et al. 2025. Placental inflammation-driven T cell memory formation promotes allergic responses in offspring via endogenous glucocorticoids. Mucosal Immunology.
Summary by Stefan Botha