In a major advance for cancer immunotherapy, researchers have developed a new type of CAR T cell that only activates its tumor-killing machinery when it encounters specific features of the tumor microenvironment (Figure 1). This approach greatly improves the precision and safety of CAR T therapy against solid tumors.
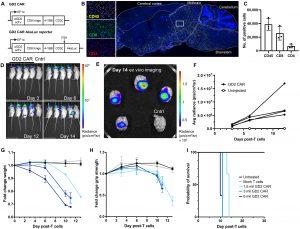
Figure 1: GD2 CAR T cells cause fatal neurotoxicity in immunodeficient mice. (A) Design of lentiviral αGD2CAR construct and αGD2CAR AkaLuc reporter. (B) Representative image of CD45 histological staining of NSG mouse brains treated with primary human αGD2CAR T cells. Left-hand panels show zoom of white box region of interest. Comparative analysis of CD4- and CD8-positive brain-infiltrating populations of primary human GD2CAR T cells. (C) Quantification of brain-infiltrating cells (n = 3 mice). (D) Time course of GD2 CAR T cell expansion via BLI of AkaLuc expressing primary T cells (n = 4 mice) or control-injected mice (Cntrl, mock T cell not expressing AkaLuc). All mice are in indicated order as in the top-left panel. (E) Ex vivo imaging of mouse brains on day 14 end point by BLI. (F) Quantification of brain-specific CAR T cell expansion. Quantification of mouse weight (G), grip strength (H), and overall survival (I) for a dose titration of GD2 CAR T cells (n = 3 mice per group). mil, million. Data are means with individual data points (C) or means ± SD [(F) to (I)].
Solid tumors often express the same antigens found on healthy tissues, which can lead conventional CAR T cells to cause dangerous “on-target, off-tumor” side effects. To solve this problem, the team focused on P-selectin, an adhesion molecule that is abundant on the blood vessels feeding many solid tumors but largely absent in normal organs. They engineered a synthetic Notch receptor that senses P-selectin and only then turns on the CAR gene. In this way, the CAR T cells act like an “AND” gate, recognizing both the tumor antigen and the presence of P-selectin before unleashing their full cytotoxic power.
Preventing Neurotoxicity While Maintaining Potency
The researchers tested a high-affinity GD2 CAR that in previous models caused fatal neurotoxicity by attacking GD2-positive cells in the brain. When using their P-selectin-gated design, the CAR remained off in healthy brain tissue. Mice treated with these gated cells showed no signs of neurological damage and maintained stable body weight. In contrast, standard GD2 CAR T cells infiltrated the brain and led to the mice’s death.
Strong Antitumor Response in Neuroblastoma Models
In mouse models of neuroblastoma engineered to express human P-selectin, a single dose of the gated GD2 CAR T cells slowed tumor growth and extended survival without any detectable off-tumor effects. If P-selectin was removed from the tumor environment, the cells remained inactive and the tumor grew unchecked. This clear on-off behavior confirms that the circuit responds exactly where it is needed.
Improved T Cell Fitness and Longevity
Beyond safety, the new CAR T cells displayed a more “youthful” profile. They retained markers of memory-like T cells and showed lower levels of exhaustion markers when compared to conventional CAR T cells. Metabolic tests revealed that these cells had greater mitochondrial reserve, a key trait for long-lasting antitumor activity even after repeated encounters with tumor cells.
A Flexible Platform for Multiple Targets
To demonstrate the versatility of their system, the researchers also applied the same P-selectin-triggered approach to CAR T cells targeting CD19 in solid tumor settings. These CD19-gated cells similarly outperformed standard CD19 CAR T cells by only activating in the tumor microenvironment and sparing healthy tissue. In mixed-cell experiments where P-selectin and CD19 were on different cells, the gated T cells still successfully found and eliminated the tumor cells nearby.
Towards Safer, More Effective Solid Tumor Therapies
This “microenvironment-sensing” CAR T cell strategy promises to open new doors in the fight against solid cancers. By using features of the tumor vasculature as a precise on-switch, it is now possible to harness highly potent CARs without risking collateral damage to healthy organs. Future work will explore how widely P-selectin is expressed across different tumor types and will aim to move these promising cells into clinical trials. If successful, patients may soon benefit from CAR T therapies that are both safer and more effective than ever before.
Journal article: Vogt, K.C. et al. 2025. Microenvironment-Actuated CAR T Cells Improve Solid Tumor Efficacy Without Toxicity. Sci. Adv.
Summary by Gaurang Telang










