Since the outbreak of COVID-19, researchers have struggled to fully understand why some infections lead to severe, life-threatening pneumonia, while others cause only mild symptoms. A new study identifies the immune protein lipocalin 2 (Lcn2) as a major contributor to lung injury in severe cases of COVID-19 (Figure 1).
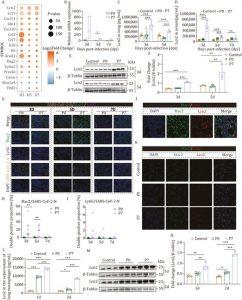
Figure 1: P7 stain infection significantly augmented the secretion of Lcn2 in macrophages. After infecting BALB/c mice with the P0 strain and P7 strain, the lung was collected at 3, 5, and 7 dpi for RNA-Seq analysis, Western blot, ELISA, and multiplex IHC staining. (A) The differential expression of genes at various time points following infection with the P7 mutant strain compared to the P0 strain. The size of the circles represents −log10(P value), while the color indicates log2(Fold Change). (B) Lcn2 mRNA expression in the lungs of infected mice was detected by qRT-PCR at 3, 5, and 7 dpi (n = 3). (C) Lcn2 protein expression in lung homogenates of infected mice was detected by ELISA at 3 and 5 dpi (n = 5). (D) Lcn2 protein expression in the serum of infected mice was detected by ELISA at 3, 5, and 7 dpi (n = 6). (E and F) Lcn2 protein expression in the lungs of infected mice was detected by Western blot at 3 and 5 dpi (n = 3). (G) Co-localization of the SARS-CoV-2 viral antigen (N protein, the fourth line) with macrophages (Mac2, the second line) and neutrophils (Ly6G, the third line) in the lungs of infected mice was assessed by multiplex IHC staining. (H) The ratio of Mac2 to SARS-CoV-2-N double-positive cells was determined (n = 9). (I) The ratio of Ly6G to SARS-CoV-2-N double-positive cells was determined (n = 9). (J) The co-localization results of lung macrophages (Mac2, the second pannel) and Lcn2 (the third pannel) were analyzed by immunofluorescence. (K) The co-localization results of sorted macrophages (Mac2, the second pannel) and Lcn2 (the third pannel) from the lungs of mice in each group (control, P0, and P7 group) were analyzed by cell immunofluorescence. (L) Lcn2 protein expression in the supernatant of macrophages sorted from the lungs of mice in each group (control, P0, and P7 group) was detected by ELISA at 1 dpi and 2 dpi (n = 3). (M and N) Lcn2 protein expression in the lungs of mice in each group (control, P0, and P7 group) was detected by Western blot at 1 and 2 dpi (n = 3). Bar = 25 μm or 10 μm. Significant differences are indicated with asterisks (*P < 0.05; **P < 0.01; ***P < 0.001; Student’s t-test or one-way ANOVA analysis).
The research offers new insights into how overactive immune responses, particularly those driven by macrophages, can escalate lung inflammation and damage. The findings may guide the development of targeted treatments for severe respiratory disease, including future SARS-CoV-2 variants.
Most mouse models of COVID-19 reflect mild or moderate disease, limiting their utility in studying severe respiratory failure. To address this gap, the researchers engineered a mouse-adapted SARS-CoV-2 strain (P7), derived from the Beta variant, that reliably caused severe pneumonia and high mortality in wild-type BALB/c mice.
Infected mice displayed extensive lung inflammation, elevated levels of inflammatory cytokines, and widespread alveolar damage—closely mimicking the clinical features of severe COVID-19 in humans.
Using transcriptomic and proteomic profiling, the team identified Lcn2 as one of the most highly upregulated proteins during infection. Produced primarily by macrophages, Lcn2 was shown to be activated via the NLRP3 inflammasome pathway, a well-known immune signalling cascade linked to inflammation.
Inhibiting NLRP3 led to a significant reduction in Lcn2 levels, confirming the pathway’s central role in regulating this potent inflammatory mediator.
Functionally, Lcn2 was found to exacerbate lung inflammation through several mechanisms:
- Stimulating endothelial cells to produce adhesion molecules like VCAM1, promoting immune cell accumulation in the lungs
- Increasing neutrophil binding to blood vessel walls
- Disrupting vascular integrity by weakening intercellular junctions
This cascade of effects ultimately resulted in greater immune cell infiltration, vascular leakage, and tissue damage—hallmarks of severe COVID-19.
The study also uncovered a mutation—W682R, near the furin cleavage site of the viral spike protein—that may enhance the infectivity and inflammatory potential of the P7 strain. This suggests a potential link between viral evolution and heightened immune activation, offering another explanation for how certain variants trigger more severe disease.
As SARS-CoV-2 continues to evolve, understanding how the immune system responds, and sometimes overreacts, remains essential for pandemic preparedness. This study offers a compelling example of how host-pathogen interactions drive disease severity and opens new avenues for targeting the inflammatory responses at the root of lung injury.
Journal article: Liu, M., et al., 2024. Lcn2 secreted by macrophages through NLRP3 signaling pathway induced severe pneumonia. Protein & Cell.
Summary by Stefan Botha










