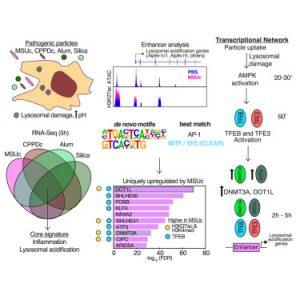
From crystal-induced arthritis to lung damage caused by airborne particles, the body’s exposure to toxic materials is a key driver of chronic inflammation. A new study has revealed how immune cells known as macrophages orchestrate their response to such particle – through two distinct transcriptional programs that govern inflammation and cellular housekeeping separately (Figure 1).
The findings offer important insights into how macrophages interpret and respond to dangerous particles such as urate crystals (MSUc), calcium pyrophosphate crystals (CPPDc), silica, and aluminum salts. These discoveries may pave the way for new therapies that target harmful inflammation without compromising the cell’s ability to clear toxic material.
Macrophages are the body’s professional clean-up crew. These long-lived immune cells patrol tissues, engulfing and digesting pathogens, dying cells, and toxic particles. Digestion happens in lysosomes—acidified organelles that break down what the cell ingests. However, when macrophages ingest toxic crystals like those found in gout or silicosis, their lysosomes can rupture, triggering strong inflammatory responses.
To explore how macrophages regulate these responses at the genetic level, the researchers exposed mouse macrophages to four types of inflammatory particles—MSUc, CPPD, crystalline silica, and aluminium salts – and analysed gene expression changes using RNA sequencing.
They discovered that particle exposure triggers two separate transcriptional programs:
- One driving inflammation through cytokine and chemokine expression
- One activating lysosomal acidification and repair, essential for proper digestion and particle clearance
To map regulatory pathways, the team turned to both mouse and human macrophages and identified two distinct signaling axes:
- The inflammatory gene program was controlled by the well-known JNK–AP-1 signaling pathway
- The lysosomal acidification program was regulated by a novel AMPK–TFEB/TFE3–DNMT3A/DOT1L axis
AMPK, an energy-sensing kinase, was shown to activate TFEB and TFE3 – transcription factors that turn on genes involved in lysosomal acidification. Using chromatin mapping, the team found that TFEB and TFE3 selectively bound genes involved in lysosome function after stimulation with MSUc.
Two additional regulators were key to this program:
- DOT1L, a histone methyltransferase, was required for activation of lysosomal genes
- DNMT3A, commonly known for DNA methylation, also played a transcriptional role here independent of its catalytic activity
The findings may hold therapeutic value in diseases such as gout, CPPD, and silicosis, where particle-driven inflammation leads to tissue damage. Targeting specific arms of this dual-response system could allow researchers to dampen excessive inflammation while preserving macrophage function.
As understanding of these distinct transcriptional programs deepens, so too does the potential for precise therapeutic interventions in particle-induced inflammatory diseases.
Journal article: Cobo., I., et al, 2025. Particle uptake by macrophages triggers bifurcated transcriptional pathways that differentially regulate inflammation and lysosomal gene expression, Immunity.
Summary by Stefan Botha