A longstanding challenge in HIV vaccine development has been teaching the immune system to generate broadly neutralizing antibodies (bnAbs) capable of targeting the virus despite its rapid mutation and immune evasion tactics. Now, a new study offers strong evidence that a stepwise, mRNA-based vaccine strategy can prime and guide immune responses in humans—marking a pivotal advance in the quest for a globally effective HIV vaccine (Figure 1).
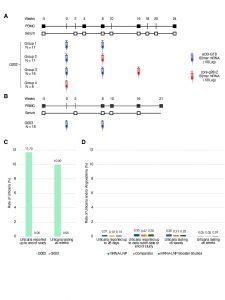
Figure 1: Trial schema and rates of urticaria compared to Moderna studies. (A) G002 schema. (B) G003 schema. (C) Rates of urticaria in G002 and G003. (D) Rates of urticaria and/or angioedema in pooled Moderna non-HIV studies described in the text. In (D), mRNA-LNP represents Moderna vaccines; Comparator represents placebo or non-mRNA vaccines included in the Moderna studies; and mRNA-LNP Booster Studies represents mRNA-1273 booster studies.
Involving nearly 80 participants across two early-phase clinical trials in North America and sub-Saharan Africa, the study demonstrates that a carefully sequenced vaccine regimen can initiate and further mature the types of immune cells needed to combat HIV. The findings, highlight the potential of using germline-targeting immunogens and mRNA platforms to develop effective, customizable immune responses.
The first trial (IAVI G002) tested a heterologous prime-boost strategy, in which participants received an initial vaccine to activate rare precursor B cells and a modified booster to guide these cells toward developing VRC01-class antibodies—early forms of bnAbs known to target conserved regions of HIV. Impressively, all participants who received both shots developed these key antibody responses, and over 80% achieved “elite” immune features linked to bnAb development.
The second trial (IAVI G003), conducted in South Africa and Rwanda, focused on the priming step. It showed that the vaccine effectively activated the desired immune cells in African participants, reinforcing its relevance for populations most burdened by HIV. The immune responses in African and North American participants were remarkably similar, supporting the vaccine’s global applicability.
Both trials utilized an mRNA platform—similar to that used in COVID-19 vaccines—which allowed for rapid development and elicited robust immune responses. The strategy also relied on “germline targeting,” a method that activates the specific, rare B cells capable of evolving into bnAb-producing cells. Subsequent doses guide these cells through a maturation process essential to developing potent antibody defences.
Although these trials weren’t designed to induce bnAbs outright, they provide compelling proof of concept for a staged vaccination approach that could eventually lead the immune system to produce them. By generating VRC01-class antibodies, the research demonstrates that key early steps in bnAb development can be successfully orchestrated in humans.
As next-generation studies are already being prepared, the hope is that continued refinement of this approach will bring the scientific community one step closer to achieving an effective, durable HIV vaccine for global use.
Journal article: J.R. Willis, et al., 2025. Vaccination with mRNA-encoded nanoparticles drives early maturation of HIV bnAb precursors in humans. Science.
Summary by Stefan Botha










