A new study sheds light on how gut-residing microbes may reprogram immune cells and trigger systemic autoimmune diseases like rheumatoid arthritis (RA) (Figure 1). The research uncovers how T cells originating in the gut undergo a transformation that enables them to migrate throughout the body and drive joint inflammation.
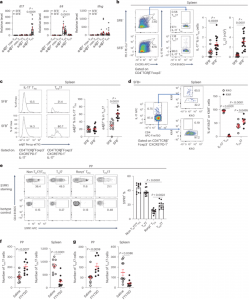
Figure 1: Splenic TFH cells of gut origin are enriched with TFH17 cells, and S1PR1 mediates the migration of TFH17 cells from PPs to spleen. a, SFB− and SFB+ K/BxN splenocytes were sorted for α4β7− or α4β7+ non-TFH and TFH cells. Il17, Il4 and Ifng transcripts were determined by RT-PCR. Data were normalized with the Hprt housekeeping gene. Non-TFH and TFH cells were further normalized to one of the SFB− non-TFH samples (value set as 1) (n = 7 mice/SFB− , and 17 mice/SFB+ , data combined from four independent assays). b, Representative plots and quantitative data of splenic TFH17 cells are shown (n = 13 mice/SFB− , and 17 mice/SFB+ , data combined from four independent assays). c, Representative plots and quantitative data show the percentages of splenic α4β7+ IL-17− TFH and α4β7+ TFH17 cells (n = 11 mice/SFB− , and 13 mice/SFB+ , data combined from three independent assays). d, SFB+ KikGR.K/BxN mice were subjected to photoconversion surgery. Three days after surgery, the PP-derived and photoconverted cells in the spleen are shown as the KikR+ population, which was gated (indicated by blue arrows) on IL-17− TFH and TFH17 cells. Representative plots and quantitative data show the percentages of KikG and KikR in IL-17− TFH and TFH17 (n = 7 mice/group, data combined from two independent assays). e, Representative plots and quantitative data show the percentages of S1PR1+ cells in non-TH17/TFH, Rorγt+ T (TH17), Rorγt− (conventional) TFH, and Rorγt+ TFH (TFH17) cell populations in PP cells from SFB+ K/BxN mice (n = 11 mice/group, data combined from three independent assays). f,g, 6- to 7-week-old and 4-week-old SFB+ K/BxN mice were treated with FTY720 for 3 (f) and 10 (g) days, respectively, before tissues were harvested for analyses. Quantitative data indicating the number of PP and splenic TFH17 cells are shown (f: n = 9 mice/saline, and 10 mice/FTY720, data combined from two independent assays; and g: n = 10 mice/saline, and 9 mice/FTY720, data combined from two independent assays). In Fig. 1, data are presented as mean values ± s.e.m. Two-way ANOVA with Sidak’s multiple comparisons test in a and d, two-tailed unpaired t-test in b, c, f and g, one-way ANOVA with Tukey’s multiple comparisons test in e.
Key Discovery: Gut-Induced T Cell Plasticity
The research focused on TFH17 cells, a hybrid immune cell that:
- Originates from T helper 17 (TH17) cells in the gut
- Adopts features of T follicular helper (TFH) cells, which assist B cells in producing antibodies
- Retains mobility and proinflammatory capabilities, unlike conventional TFH cells
How It Works
- Trigger: Harmless gut microbes, particularly segmented filamentous bacteria, stimulate TH17 cells in the small intestine.
- Reprogramming Site: In specialized lymphoid tissues called Peyer’s patches, these TH17 cells transform into TFH17 cells through a process called fate-mapping.
- Mobility & Function: These hybrid cells:
- Exit the gut and enter circulation
- Retain inflammatory activity
- Potently assist B cells—key players in RA pathology
Disease Impact in Mice
- Mice with TFH17 cells developed 4.8x more joint inflammation than mice with only conventional TFH cells.
- These hybrid cells shared gene signatures with immune cells found in the blood of people with RA, including gut-specific markers—linking findings in mice to human disease.
This study:
- Reveals how commensal gut bacteria can promote systemic autoimmune disease through immune cell reprogramming
- Points to TFH17 cells as a therapeutic target in RA—and potentially in lupus and other autoimmune disorders
- Suggests that manipulating gut-immune interactions could be a strategy to treat or prevent autoimmunity
Journal article: Fan, T., et al. 2025. Aberrant T follicular helper cells generated by TH17 cell plasticity in the gut promote extraintestinal autoimmunity. Nature Immunology.
Summary by Stefan Botha










