- Cancer Stem Cells (CSCs) are defined by three properties:
- Tumour initiation capacity,
- Self-renewal and
- Differentiation. (Ward and Dirks 2007).
- Current approaches to identify human CSCs include: phenotypic or functional purification, in vitro assays to measure self-renewal, differentiation and signaling pathways through sphere-forming assays, or in vivo assays to study self-renewal in serial recipients and analyze their histological phenotype (Figure 1).
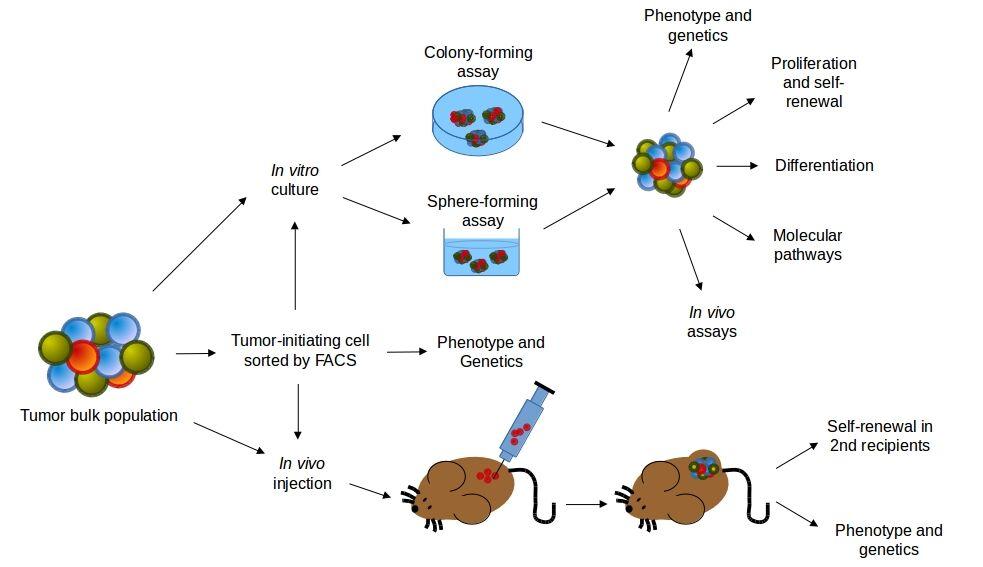
Figure 1. Experimental approaches for the study of human cancer stem cells (CSCs). Human tumours are dissociated and may be in vitro cultured or injected in vivo after phenotypic or functional purification. The self-renewal activity of CSCs can be evaluated by serially culturing in colony-forming or sphere-forming assays, or by the serial transplantations into recipient mice. CSCs should regenerate a tumour phenotipically representative of the cancer from which it was sampled. In vitro and in vivo approaches may be combined in oder to evaluate other CSCs characteristics, like differentiating potential, survival, proliferation, genetics and functionality of molecular pathways. [Ward and Dirks, 2007 by J.C. Balandrán and J. Enciso.]
- Two models try to explain tumour origins and progression of tumours.
- The cancer stem cell model (CSC) model assumes that only a very small subset of cells within the tumour population actually has the capacity to initiate and sustain tumour growth (Figure 2a).
- The stochastic model assumes that every cancerous cell has the capacity to extensively proliferate and regenerate a tumour but the probability of this event is low (Figure 2b). The model predicts that isolating discrete populations of cancer cells based on functional or phenotypic characteristics cannot enrich the tumour-initiating capacity.
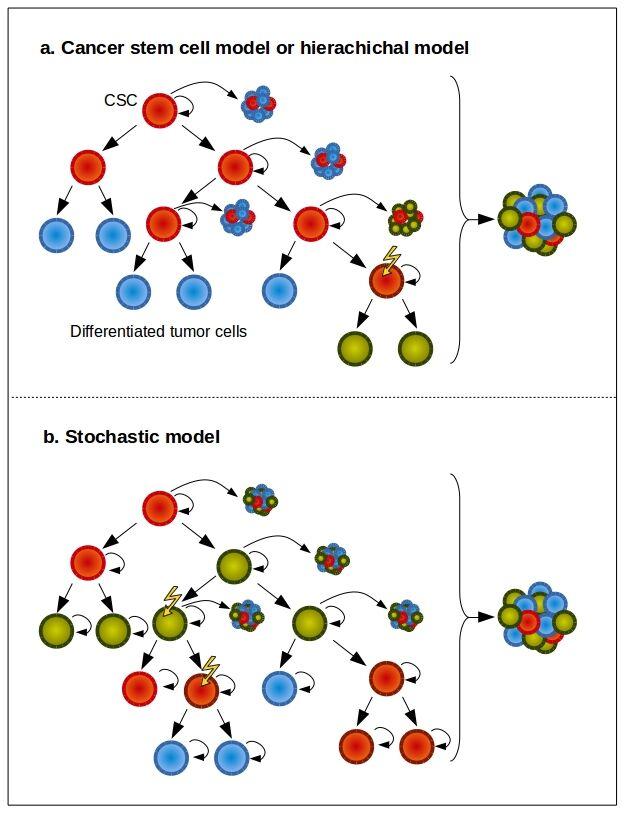
Figure 2. CSC and stochastic models of tumour growth. a) In the CSC model of tumour growth, only a subset of tumour cells has the ability for long-term self-renewal (red cells) and these cells give rise to committed progenitors with limited proliferative potential that eventually terminally differentiate. b) In the stochastic model of tumour growth, all tumour cells are equipotent and stochastically self-renew or differentiate, leading to tumour heterogeneity. In both models, new somatic mutations can generate clonal diversity, which further increases tumour heterogeneity. [Beck and Blanpain 2013, by J.C. Balandrán and J. Enciso.]
- In the hematopoietic system, it has been proposed that one intermediate HSC (pre-leukemia HSC) can harbour some, but not all, leukemia-specific mutations accumulated during a prolonged “pre-leukemic” phase (Figure 3).
- Lapidot et al. in 1994 demonstrated that cells CD34+CD38+ from acute myeloid leukemia (AML) patients could be injected into severe combined immunedeficient (SCID) mice to generate a leukemia phenotype (Lapidot et al. 1994).
- Years later, this serially transplantable population of human leukemia cells from AML patients enriched from tumour-initiating abilities was called leukemia stem cells (LSCs). Its further characterisation revealed a functional hierarchy (Bonnet and Dick 1997).
- The first identification of a cancer stem cell population in solid tumours was made in breast as CD44+CD24-/lo . Lineage, that was enriched in tumour-initiating ability when injected into NOD/SCID mice and serially transplanted into recipients.
- Analysis of tumours demonstrated the presence of a heterogeneous population of cells similar to that of the original tumour (Al-Hajj et al. 2003).
- Additional markers have been proposed to make the difference between CSCs from normal cells, CD133 is one of them, this surface marker is expressed in the minority of cultured tumour cells. In vitro, a subfraction of CD133+ cells formed tissue spheres whereas CD133– did not.
- Like normal stem cells, CSC can be isolated on the basis on their ability to efflux Hoechst33342 (a DNA-binding fluorescent dye).
- In HSCs, Hoechst is excluded from the stem cell population within a short time after incubation through export protein pumps. Hoechsttlow/- fraction included stem cells and is also called side population (SP). (Goodell et al.).
- On the other hand, aldehyde dehydrogenase (ALDH) is also found in stem cells and can be useful to identify their simile population in cancer. ALDH+ cells have been isolated from cancer breast, liver, colon, pancreas, prostate, lung, ovarian and leukemia where stemness comes together with high enzyme activity.
- The identification of CSCs based on their metabolic state is a promising research field still in progress where CSCs maintain low ROS levels exhibiting redox patterns that are similar to the corresponding normal stem cells.
- Anticancer therapies targeting the bulk tumour do not eliminate stem cells and it may lead to disease recurrence and progression.
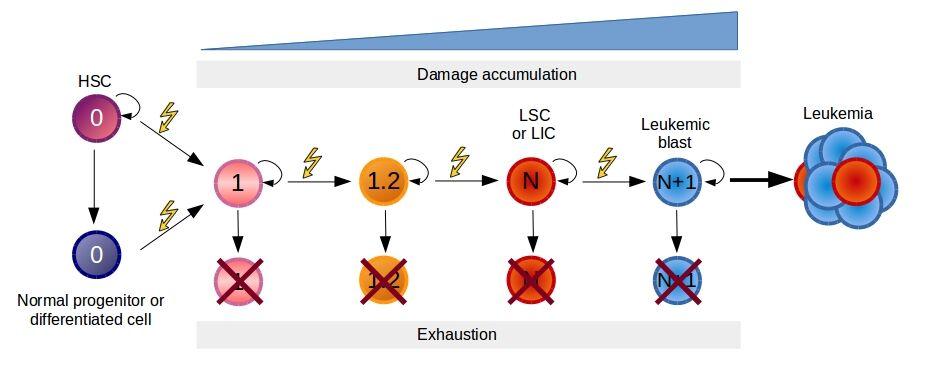
Figure 3. Model of pre-leukemic evolution of leukemia. Malignant transformation is a process requiring a progressive accumulation of mutations. Normal hematopoietic stem and progenitor cells are represented as cells with absence of mutations or damage. The first mutation may target a HSC or a more differentiated cell inducing the retention or acquisition of the self-renewal potential. Every time a new mutation appears, there is the possibility that results lethal for the cells or after a period of derives in clone depletion due to immune surveillance or competition with normal hematopoiesis. Eventually, after the accumulation of sufficient mutations (N) a leukemic stem cell (LSC) or leukemic initiating cells (LIC) is generated. The resultant leukemic cell loses the capability to differentiate into multiple cellular lineages and leads to the progression of the disease. [Corces-Zimmerman and Majeti, 2014 by J.C. Balandrán and J. Enciso.]
Quiz
References and Links
- Al-Hajj, Muhammad, Max S Wicha, Adalberto Benito-Hernandez, Sean J Morrison, and Michael F Clarke. 2003. “Prospective Identification of Tumorigenic Breast Cancer Cells.” Proceedings of the National Academy of Sciences of the United States of America 100 (7): 3983–88.
- Beck, Benjamin, and Cédric Blanpain. 2013. “Unravelling Cancer Stem Cell Potential.” Nature Reviews. Cancer 13 (10): 727–38.
- Bonnet, D, and J E Dick. 1997. “Human Acute Myeloid Leukemia Is Organized as a Hierarchy That Originates from a Primitive Hematopoietic Cell.” Nature Medicine 3: 730–37.
- Corces-Zimmerman, M R, and R Majeti. 2014. “Pre-Leukemic Evolution of Hematopoietic Stem Cells: The Importance of Early Mutations in Leukemogenesis.” Leukemia 28 (12). NIH Public Access: 2276–82.
- Goodell, M A, K Brose, G Paradis, A S Conner, and R C Mulligan. 1996. “Isolation and Functional Properties of Murine Hematopoietic Stem Cells That Are Replicating in Vivo.” The Journal of Experimental Medicine 183 (4): 1797–1806.
- Guzman, M L, S J Neering, D Upchurch, B Grimes, D S Howard, D A Rizzieri, S M Luger, and C T Jordan. 2001. “Nuclear Factor-kappaB Is Constitutively Activated in Primitive Human Acute Myelogenous Leukemia Cells.” Blood 98 (8): 2301–7.
- Jaiswal, Siddhartha, Catriona H M Jamieson, Wendy W Pang, Christopher Y Park, Mark P Chao, Ravindra Majeti, David Traver, Nico van Rooijen, and Irving L Weissman. 2009. “CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis.” Cell 138 (2): 271–85.
- Lapidot, T, C Sirard, J Vormoor, B Murdoch, T Hoang, J Caceres-Cortes, M Minden, B Paterson, M A Caligiuri, and J E Dick. 1994. “A Cell Initiating Human Acute Myeloid Leukaemia after Transplantation into SCID Mice.” Nature 367 (6464): 645–48.
- Ward, Ryan J, and Peter B Dirks. 2007. “Cancer Stem Cells: At the Headwaters of Tumor Development.” Annual Review of Pathology 2 (January): 175–89.
- Guzman, M. & Allan, J. Leukemia Stem Cells in Personalized Medicine. Stem Cells. 32(4): 844–851. (2014)
- Zong, H. et al. A Hyperactive Signalosome in Acute Myeloid Leukemia Drives Addiction to a Tumor-Specific Hsp90 Species. Cell Reports. (13): 2159–2173. (2015)










