- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final Outcome
- References
- Evaluation - Questions & answers
- MCQ
Patient Presentation
A 31 year old caucasian male presented to the HIV clinic complaining of recent onset of fever, vomiting, joint pain, muscle aches, sore throat and a fine red rash on his face and arms. All symptoms had worsened since they first started 2 days ago and seemed to be aggravated shortly after taking his daily ARVs.
He is known to the clinic having been on ARV’s since testing positive three years ago. He is considered a compliant patient always keeping appointments and having maintained an undetectable viral load since starting treatment. His most recent CD4 count was 393 cells/µL having been checked five weeks prior to this visit.
He has no recent travel history and is unaware of any contact with anyone else presenting with similar symptoms.
History
Patient was tested HIV positive three years ago at age 28. His CD4 count was 185 cells/µL and viral load measurement was 20800 copies/mL. He was started on first line ARV therapy – Stavudine (d4T), Lamivudine (3TC) and Efavirenz (EFV), which he continued to take until his last appointment 5 weeks ago.
At this appointment he noted that he had gained 3kg over the last three months. Although he was not particularly concerned by the weight gain he was finding that his shirts were feeling uncomfortably tight around the neck and it felt like he was developing a bulge at the back of his neck. He also noted that his face was becoming gaunt and his cheekbones were starting to stick out.
After a thorough examination and investigations this was considered to be due to d4T, which is known to cause lipodystrophy as one side effect. Typically presenting with the development of a buffalo hump on the back of the neck and facial lipodystrophy. The d4T was stopped and replaced with Abacavir (ABC). The rest of his regimen remained unchanged as his CD4 count had remained at 393 cells/µL and his viral load had remained undetectable.
The patient had been asked to return in three months for his usual follow up unless he developed any complications. Now 5 weeks later the patient has returned to the clinic with a two-day history of fever, nausea, vomiting, sore throat, general malaise and a fine maculopapular rash on his face an arms.
Past Medical History
- Nil
Past surgical History
- Appendectomy at age 15yrs
Family History
- Father has hypertension, on treatment
- Mother is asthmatic, on treatment as needed
Allergies
- None known
Medication
- Cotrimoxazole
- 3TC, EFV and ABC
- Multivitamins
Travel History
- Nil
Social History
- Lives with partner of 2 years in a house with full amenities
- Employed as an administrative assistant
Differential Diagnosis
- Food allergy
- Anaphylaxis
- Food poisoning
- Adverse reaction to ARV’s
- Hypersensitivity to ARV’s
Appearance
- Ill looking Caucasian male of average height and weight
- Awake, alert, and co operative
- GCS 15/15
Vitals
- Temperature: 38.0°C
- Blood pressure: 135/78
- Heart rate: 105
- Respiratory rate: 20
General
- Mild pallor
- No jaundice
- Cervical lymphadenopathy
- No oedema
Head and Neck
- Fine maculopapular rash on face
- Red, slightly oedematous pharynx
- Sunken cheek bones
- Buffalo hump on the back of the neck
Chest
- Trachea centrally located.
- No wheeze
- Lung fields clear bilaterally
- No other added breath sounds
Cardiovascular
- No raised JVP
- Normally placed apex beat
- S1 and S2 heart sounds present, no gallop, no murmurs.
- No abnormalities detected
Abdomen
- Not distended
- Soft, with generalised tenderness
- Bowel sounds present
- No abnormalities detected
- No organomegally
Neurological
- Normal level of consciousness
- Gait normal
- Power 5/5 globally
- Tone normal globally
- Reflexes 2/4 for both upper and lower limbs
- No signs of peripheral neuropathy
Dermatological
- Fine maculopapular rash on face and arms
| Examination | Value | Normal Limits |
|---|---|---|
| Initial presentation | ||
| WBC | 4.8 | (4-12 x109/L) |
| HB | 14.2 | (12.1-15.2 g/L) |
| Platelets | 217 | (140-450 x109/L) |
| CRP | 15 | (0-8mg/l) |
| NA | 137 | (135-147 mmol/L) |
| K | 3.9 | (3.3-5.0 mmol/L) |
| Cl | 100 | (99-103 µmol/L) |
| C02 | 19 | (18-29 mmol/L) |
| Urea | 6 | (2.5-6.4 mmol/L) |
| Creatinine | 108 | (62-115 mmol/L) |
| Corrected Calcium | 2.16 | (2.1-2.6mmol/l) |
| Phosphate | 1.4 | (1.0-1.5 mmol/l) |
| Magnesium | 1.1000000000000001 | (0.8-1.3) |
| Total protein | 73 | (60-80g/L) |
| Albumin | 41 | (35-50g/L) |
| Total Bilirubin | 6 | (0-10 µmol/L) |
| Direct Bilirubin | 2 | (0-4 µmol/L |
| ALP | 103 | (30 - 150 U/L) |
| Gamma GT | 20 | (11 - 51 U/L) |
| ALT | 20 | (5 - 35 U/L) |
| AST | 22 | (5 - 35 U/L) |
| CD4+ | 396 | |
| Viral load | (Copies per ml) | |
| Blood Culture | negative |
Discussion
The patient in our case study has an Abacavir (ABC) hypersensitivity reaction. This is a multi-organ systemic reaction that that can occur in up to 8% of HIV-infected patients initiated on this therapy. The reaction resembles a delayed hypersensitivity reaction, which can cause life-threatening complications with continued use during initial therapy, or immediate and potentially fatal reactions in people who are re-challenged following a prior hypersensitivity reaction.
The presentation of a Hypersensitivity Reaction
The incidence of ABC hypersensitivity is ∼3.7%, with an estimated 1 case in every 25 patients who receive the drug. Other reports have documented an occurrence of 5-8% among ABC-treated patients. It predominantly affects Caucasians and Asians while the risk among people of African descent is unknown and is approximately 2% in African Americans. Typically, almost all abacavir hypersensitivity reactions occur within the first 4-6 weeks of therapy and are reversible with discontinuation of use, however failure to recognize the reaction has been associated with fatalities when therapy was continued despite progressive symptoms.
Clinical Presentation
ABC hypersensitivity involves a host of general non-specific signs and symptoms including fever, nausea, vomiting, diarrhoea or abdominal pain, rash, sore throat, fatigue, malaise and non productive cough, as observed in our case study. In contrast to the more prominent rash that can develop with sulfonamides or non-nucleoside reverse transcriptase inhibitors (NNRTIs), the rash associated with ABC is often mild and not the predominant symptom. Typically the constellation of symptoms associated with ABC hypersensitivity frequently mimic those of viral illnesses, such as influenza. Individuals may also experience certain respiratory symptoms that can be mistaken for pneumonia such as cough and pharyngitis. A key component of ABC hypersensitivity is the accentuation of symptoms within hours of taking each dose of the drug and the escalation of symptoms with each subsequent dose.
Although much emphasis is placed on the importance of recognizing ABC hypersensitivity, it is also important not to overdiagnose and to obtain supporting empirical evidence. Of note, once ABC has been discontinued, because of presumed hypersensitivity, the patient cannot be treated with ABC again. Thus, when abacavir is discontinued prematurely or without adequate assessment of symptoms, therapeutic options may be lost. Therefore it is important that patients are assessed carefully for risk of developing ABC hypersensitivity prior to starting the regimen.
If hypersensitivity to ABC occurs, the drug should be discontinued and supportive care provided. After withdrawal, symptoms usually resolve within a few days.
HLA-B*57:01 as a genetic risk marker
Since 2000, studies have been conducted on ABC hypersensitivity using various genetic markers and found that individuals who possess the Human Leukocyte Antigen (HLA)-B*57:01 allele are more likey to develop an ABC hypersensitivity syndrome. It is thus recommended that patients who are to be provided with ABC, have an HLA-B*57:01 screen is done. HLA-B*5701 is an allele of the multi-allelic HLA class I HLA-B locus that codes for cell surface glycoproteins that present antigens to T-cell receptors, leading to induction of immune responses. The HLA-B*57:01 prevalence varies across populations, with the greatest numbers reported within the European white population (about 7%). We will discuss below why hypersensitivity occurs with the specific HLA type and not others.
Understanding Abacavir activity
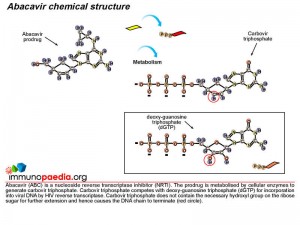
First, lets understand the mode of action of ABC as an antiretroviral drug. ABC is a prodrug formulated nucleoside reverse transcriptase inhibitor (NRTI). NRTIs are made of molecules that, once in the cell, are converted to structures similar to DNA bases which become part of the DNA strand transcribed by HIV polymerase (or reverse transcriptase) from HIV RNA. These medications, however, block the DNA from further elongation.
Once inside a cell, ABC is converted to the active drug, carbovir triphosphate, by cytosolic enzymes. During reverse transcription mediated by viral reverse transcriptase, carbovir triphosphate competes with deoxy-guanosine triphosphate (dGTP) for incorporation into viral DNA and once incorporated inhibits HIV replication by terminating the DNA chain.
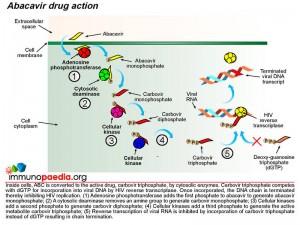 Metabolism of ABC to the active drug is achieved through a series of cellular biochemical processes. Firstly adenosine phosphotransferase adds a phosphate group to ABC to produce abacavir monophosphate. A cytosolic deaminase then removes an amine group to produce carbovir monophosphate. The process continues and cellular kinases add a second phosphate to produce carbovir diphosphate followed by addition of a third phosphate group to produce the active metabolite, carbovir triphosphate. In this form ABC competes with dGTP to inhibit the reverse transcription of HIV RNA.
Metabolism of ABC to the active drug is achieved through a series of cellular biochemical processes. Firstly adenosine phosphotransferase adds a phosphate group to ABC to produce abacavir monophosphate. A cytosolic deaminase then removes an amine group to produce carbovir monophosphate. The process continues and cellular kinases add a second phosphate to produce carbovir diphosphate followed by addition of a third phosphate group to produce the active metabolite, carbovir triphosphate. In this form ABC competes with dGTP to inhibit the reverse transcription of HIV RNA.
What role does HLA-B*57:01 play?
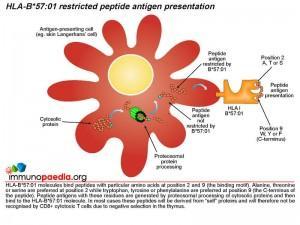
The role of HLA class I molecules is important in cell-mediated immunity since they function as antigen presenting molecules to CD8+ cytotoxic T cells. The source of the antigen is short peptide fragments of around 9-11 amino acids long derived from cytosolic proteins or in the case of dendritic cells they can be cross-presented on both HLA class I and II molecules via phagocytosed proteins. HLA class I genes occur at three genetic loci on chromosome 6 in humans (HLA-A, B and C) and a large number of allelic variants have been identified worldwide. HLA class I molecules encoded by different alleles primarily differ in their peptide binding specificity. It is known that HLA-B*57:01 molecules preferentially bind peptides with alanine, threonine or serine at position 2 while tryptophan, tyrosine or phenylalanine is preferred at the C-terminal position 9. Peptide antigens with these residues are generated by cleavage of cytosolic proteins by the proteosome in the cytoplasm and imported into the endoplasmic reticulum where they bind to newly synthesised HLA-B*57:01 molecules that are exported via the Golgi body to the cell surface for presentation to CD8+ cytotoxic T cells. In most cases these peptides will be derived from “self” proteins and will therefore not be recognised by the T cell receptor (TCR) of CD8+ cytotoxic T cells due to negative selection in the thymus. In HIV-infected individuals who carry the HLA-B*57:01 gene and who are treated with abacavir, an autoimmune hypersensitivity reaction involving CD8+ cytotoxic T cells can develop. Tissue damage as a result of CD8+ cytotoxic cells has been reported from biopsies of inflamed skin from hypersensitive patients that demonstrate a marked infiltration of CD8+ T-cells.
How is abacavir involved?
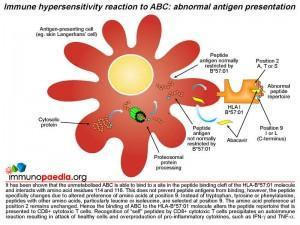
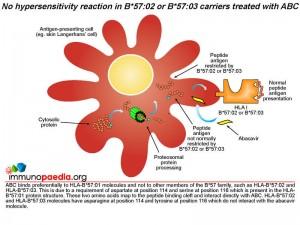
It has been shown that unmetabolised abacavir prodrug is able to bind to a site in the peptide binding cleft of the HLA-B*57:01 molecule and interacts directly with aspartate at position 114 and serine at position 116. The presence of abacavir does not prevent peptide antigens from binding to the HLA molecule, however, the peptide specificity is altered. A preference of other amino acids, particularly leucine and isoleucine, at position 9 instead of tryptophan, tyrosine or phenylalanine is evident. The amino acid preference at position 2 remains unchanged. Abacavir bound to HLA-B*57:01 molecules alters the peptide repertoire that is presented to CD8+ cytotoxic T cells. Many of the abnormal peptides that now bind HLA- B*57:01 are derived from “self” proteins. Recognition of “self” peptides by circulating CD8+ cytotoxic T cells precipitates an autoimmune reaction resulting in attack of healthy cells and overproduction of pro-inflammatory cytokines, such as IFN-gamma and TNF-alpha.
 Does abacavir bind to other HLA-B*57 molecules?
Does abacavir bind to other HLA-B*57 molecules?
Abacavir has a specificity for HLA-B*57:01 molecules and does not bind to other members of the B*57 family, such as HLA-B*57:02 and HLA-B*57:03. This is due to a requirement of aspartate at position 114 and serine at position 116 which is present in the HLA-B*57:01 protein structure. These two amino acids map to the peptide binding cleft and interact directly with the abacavir molecule. HLA-B*57:02 and HLA-B*57:03 molecules have asparagine at position 114 and tyrosine at position 116 which do not interact with the abacavir molecule.
What do the CD8+ cytotoxic T cells do that results in hypersensitivity?
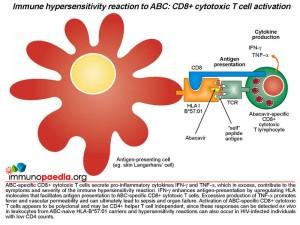
The activated CD8+ T cells that are specific to drug-modified peptides derived from the protein-ABC conjugate have been shown to secrete gamma-interferon (IFN-g) and tumour necrosis factor-alpha (TNF-a), which are pro-inflammatory cytokines that contribute to the symptoms and severity of the immune hypersensitivity reaction. Gamma-interferon enhances antigen-presentation by upregulating the numbers of HLA molecules on antigen-presenting cells that could promote further activation of CD8+ T cells. It is noted that severe skin rashes develop in most hypersensitivity reactions and may relate to the large numbers of Langerhans’ cells present on the skin that may be participating in the activation of large numbers of CD8+ T cells responding to “self” skin antigens now presented by HLA-B*57:01 molecules. Large doses of TNF-alpa promote fever and vascular permeability that can lead to sepsis and organ failure. Notably, it is thought that abacavir-specific CD8+ T cells are activated independently of CD4+ T cells, which is why patients who are severely immunocompromised with low CD4 counts can develop these reactions. This is taken to be a result of ABC interacting directly with surface and intracellular MHC molecules.
Download images for this case
Treatment
In our patient, all ARV medication and bactrim was stopped and supportive care was provided until the symptoms resolved. Although he had tolerated bactrim well since initially starting ARVs it was also stopped at this time as it was not clear what was causing the reaction. Genetic screening was conducted for a suspected ABC hypersensitivity reaction. He was found to be positive for HLA-B*5701, which provided the supporting evidence for the ABC hypersensitivity reaction.
Although ideally an HLA screen should have been performed before initiating ABC, it was thought reasonable by the treating clinician to initiate therapy with abacavir along with appropriate clinical counseling and monitoring for any signs of hypersensitivity.
Download images for this case
Final Outcome
After withdrawal of ABC, EFV, 3TC and bactrim all symptoms resolved within a week. The patient was then restarted on bactrim, he remained symptom free and two weeks later was started on Truvada and EFV. Patient has been on this regimen for 8 months with no side effects noted. His CD4 count has continued to increase moderately and viral load has remained suppressed.
Download images for this case
References
Illing PT et al. (2012) Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012 Jun 28;486(7404):554-8.
Ostrov DA et al. (2012) Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire., Proc Natl Acad Sci U S A. 2012 Jun 19;109(25):9959-64.
Norcross MA et al. (2008) Abacavir induces loading of novel self-peptides into HLA-B*57:01: an autoimmune model for HLA-associated drug hypersensitivity., AIDS. 2012 Jul 17;26(11):F21-9.Immunity. 2008 Jun;28(6):822-32.
Chessman D et al. (2008). Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. Jun;28(6):822-32.
Yuen GJ, Weller S, Pakes GE. (2008). A review of the pharmacokinetics of abacavir. Clin Pharmacokinet. 47(6):351-71.
Phillips EJ, Mallal SA. (2009). HLA and drug-induced toxicity. Curr Opin Mol Ther. Jun;11(3):231-42.
Phillips
Chaponda M, Pirmohamed M. (2011). Hypersensitivity reactions to HIV therapy. Br J Clin Pharmacol. May;71(5):659-71.
Carolino, F., Santos, N., Piñeiro, C., Santos, A. S., Soares, P., Sarmento, A., & Cernadas, J. R. (2017). Prevalence of abacavir-associated hypersensitivity syndrome and HLA-B*5701 allele in a Portuguese HIV-positive population. Porto Biomedical Journal, 2(2), 59–62.
Hewitt, R. G. (2002). Abacavir hypersensitivity reaction. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 34(8), 1137–1142.
Limou, S., Winkler, C. A., & Wester, C. W. (2019). HIV pharmacogenetics and pharmacogenomics: From bench to bedside. In Genomic and Precision Medicine (pp. 185–222). Elsevier.
Bell, C. C. et al. (2013) “T-cells from HLA-B*57:01+ human subjects are activated with abacavir through two independent pathways and induce cell death by multiple mechanisms,” Chemical research in toxicology, 26(5), pp. 759–766.
Download images for this case
Evaluation – Questions & answers
What is the diagnosis?
What is/are the main predisposing factors for this condition?
What are the most common presenting signs and symptoms seen in this reaction?
What is the mechanism of the hypersensitivity reaction to abacavir?
What are the symptoms due to CD8+ cytotoxic T cell activation
What makes it most likely that abacavir-specific CD8+ T cells are activated in a CD4+ T cell independent manner?
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case