“Wildling mice: A new preclinical tool to study human disease
Wildling mice combine the low genetic diversity of inbred laboratory mice with a wild mouse microbiota harboring bacteria, fungi, and viruses. This generates a more translationally relevant animal model of human disease for studying host-microbiota interactions, as well as for predicting the outcomes of human clinical trials.” Source Nobs & Elinav Science 2019)
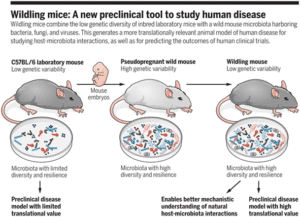
Murine models are one of the most used immunological tools, often used in pre-clinical research. Though widely used mice do not recapitulate human immunology, where in some cases favourable pre-clinical mice studies fail in phase 1 human trials. Low microbial diversity of laboratory (lab)-mice is suggested to be one of the main contributors to the discrepancy between lab-mice and humans. Rosshart et al., thus developed a wildling mouse model that genetically resembles laboratory mice and also possess microbiomes similar to wild mice.
Researchers developed wildling mice by transferring embryos from lab-C57BL/6 mice to wild mice, this enabled naturally occurring microbiome to exert effects at all stages of murine development. 16S rRNA analyses of the skin, gut and vaginal microbiome showed that wilding mice have similar microbiome and microbial -bacterial and viral- diversity as wild mice. Immunophenotyping of the gut, skin, vagina, liver and spleen using CYTOF and immune cell gene expression analysis, showed that wilding mice were more similar to wild mice than lab-mice.
Finally, to determine if wildling mice recapitulate human pathology better than lab-mice, Rosshart et al., conducted pre-clinical studies in wildling mice that passed in lab-mice but failed in humans. One of the pre-clinical studies they performed was testing the CD28-superagonist monoclonal antibody (CD28SA mAb). CD28SA mAb targets the adaptive immune by efficiently expanding regulatory T cells (Tregs), and is therapeutically active in murine autoimmune diseases. Using wildling mice, Rosshart et al., showed that CD28-SA mAb therapy in wildling mice did not expand Tregs but instead activated cells resulting in higher expression of pro-inflammatory cytokines (IFN-g, TNF, IL-6, IL-1B and IL-2) and IL-4 than lab-mice.
These findings, suggest that wildling mice are a better model for pre-clinical research as they closely resemble immune responses that happen in humans. Additionally, the presence of naturally occurring microbiome from gestation to adult development, shapes immune responses that are more similar to wild mice than laboratory mice.
Journal Article: Rosshart et al., 2019. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science
Article by Cheleka AM Mpande