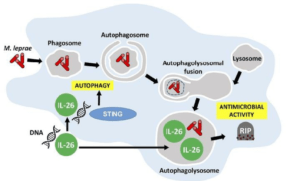
Graphical abstract demonstrating the antimicrobial activity of IL-26 against intracullar bacteria like M.leprae: IL-26 is able to colocalize with M.leprae in infected human monocyte-derived-macrophages. IL-26 may also promotes intracellular killing by promoting phagolysosomal fusion. By binding to the DNA of dead cells, IL-26 allows the cytoplasmic DNA receptor STING to bring about autophagy. (Source: Dang et al., JCI 2019)
Th17 cells majorly contribute to fighting off extracellular bacteria and are one of the main producers of the pro-inflammatory cytokine IL-26. Research by Dang et al., utilised the human leprosy model to showcase the ability of IL-26 to provide protection against intracellular bacteria. This research opens up the possibility for Th17 cells to also participate in immune defence mechanisms against not only extracellular, but also intracellular bacteria.
Gene expression profiling was done on leprosy skin lesions and showed that there were higher levels of IL26 mRNA in patients with self-limited tuberculoid leprosy (T-lep), in comparison to patients with disseminated leprosy (L-lep). Immunohistochemical analysis showed that IL-26 protein expression was abundant throughout the granuloma of T-lep lesions while its expression was close to non-existent in L-lep lesions. When colocalisation experiments were analysed in the lesions from T-lep and L-lep patients , colocalisation of IL26 with both CD4+ and CD8+ T-cells was greater in T-lep lesions than L-lep lesions.
Axenic culture of M.leprae with recombinant IL-26 protein showed that IL-26 is able to bind to the surface of the bacterium. Through colocalisation and direct binding of IL-26 to bacteria, they saw two mechanisms through which it functions in order to combat intracellular bacteria. Firstly, Dang et al., showed decreased viability of M.leprae after 3 days of IL-26 treatment. Using a colony forming unit assay, IL-26 was also observed to inhibit the growth of Mycobacterium tuberculosis H37Ra in axenic culture. This antimicrobial activity was taken to be dependent on IL-26’s native structure since denaturing the protein was not seen to produce the same result.
Secondly, the authors went on to confirm the antimicrobial functions of IL-26 by demonstrating that IL-26 treatment was able to induce autophagy in human monocyte-derived-macrophages that were infected with Staphylococcus aureus. Induction of autophagy by IL-26 is dependent on the cytoplasmic DNA receptor STING. This is supported by the fact that IL-26 is able to bind to DNA from dying cells in in-vitro cultures and traffic this DNA to activate STING. Furthermore, STING activation triggers autophagy in mycobacteria-infected macrophages. These discoveries warrant the analysis of protective immune responses by IL-26 in vaccine studies for Tuberculosis since Th1 responses, which are taken to be key for intracellular bacteria, have not been sufficient against intracellular mycobacteria.
Journal Article: Dang et al., 2019. IL-26 contributes to host defense against intracellular bacteria. Journal of Clinical Investigations.
Article by Vanessa Mwebaza Muwanga