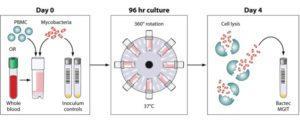
Schematic of the MGIT mycobacterial growth inhibition assay method. Whole blood or cell culture medium containing the appropriate concentration of PBMCs (or mouse splenocytes in mouse studies) is inoculated with an equal volume of mycobacteria at a low MOI (∼1 CFU/10,000 PBMCs). Cultures are incubated in 2-ml tubes at 37°C for 96 h, with 360° rotation. Cells are then lysed to remove red blood cells and/or to release intracellular mycobacteria. The lysate is inoculated into Bactec MGITs. The tubes are placed in the Bactec 960 system and incubated at 37°C until positivity is detected by fluorescence. On day 0, direct-to-MGIT viability controls are set up by directly inoculating MGIT tubes with the same volume of mycobacteria as the samples. The TTP data are converted to log10 CFU values using a standard curve, and the final values are expressed as absolute CFU values or values relative to the control value, indicating the amount of growth inhibition that occurred during the culture period. Source: Michael J. Brennan et al. Clin. Vaccine Immunol. 2017
Researchers led by Tom HM Ottenhof and Mihai Netea (Joosten et al., 2018), aimed to determine the effect of “trained immunity” on mycobacterial growth, particularly in mycobacterial growth inhibition assays (MGIAs). Researchers focused on trained immunity, as recent studies have shown that epigenetic reprogramming of monocytes may also contribute to protection.
**Read our Breaking news article on the effects of BCG-induced trained immunity on viral infection.
MGIAs measure the ability of immune cells to inhibit of growth of mycobacteria, such as Mycobacterium bovis from the Bacille Calmette-Guérin (BCG) vaccine or Mycobacterium tuberculosis. Despite, being a useful tool for detecting the capacity of individuals to restrict mycobacterial growth, the underlying immunological mechanisms for bacterial growth in MGIAs are still elusive. Studies have suggested that monocyte/lymphocyte ratios, polyfunctional CD4 T cells and even iron metabolism are associated with mycobacterial growth. However, most of these studies have not conclusively demonstrated the immune mechanisms.
Joosten et al., observed increased mycobacterial growth inhibition in recently M.tb exposed individuals compared to individuals that were either recently BCG vaccinated, latently M.tb infected or TB cases. This increased mycobacterial inhibition was associated with increased proportions of TNF-α, IL-1β and IL-6, cytokines previously associated with trained immunity.
Unlike other studies, researchers observed no association between mycobacterial growth control and Th1-cytokine producing cells. Interestingly, they observed an association between mycobacterial growth inhibition and non-classical CD14=dim monocytes producing CXCL10 (and CXCL9). This reduced mycobacterial growth required the presence of CD4+ central memory and not effector memory bulk T cells. Central memory CD4 T cells, express high proportions of CXCR3, the chemokine receptor for CXCL10, as well as CXCL9 and CXCL11 chemokines. This finding suggests that the CXCL10/CXCR3 axis is critical for BCG control, reflecting a link between innate and adaptive immunity, a hypothesis that still requires validation.
Journal Article: Joosten et al., 2018. Mycobacterial growth inhibition is associated with trained innate immunity. Journal of Clinical Investigations.
Article by Cheleka AM Mpande












