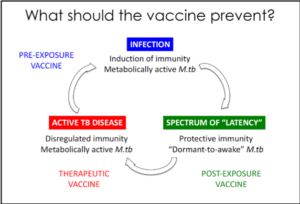
Mycobacterium tuberculosis (M.tb) causes the world’s deadliest infectious disease and is characterized by an increasing antimicrobial resistance. Bacille Calmette-Guérin (BCG) vaccination is the most widely used in the world but its efficacy against M.tb infection is variable and does not guarantee the prevention of TB infection and or the progression to TB disease (Roy et al, BMJ 2014). New preventive tools are needed to end TB epidemy. The new TB vaccines are designed to prevent TB infection, TB latency and active TB disease.
The future vaccination against TB will likely be based on heterologous prime-boost strategies. These strategies consist of subsequent vaccinations with new vaccine candidates following primary BCG vaccination at birth. Different vaccine candidates based on the concepts of delivery by viral vector, specific antigens with adjuvants, killed or live-attenuated mycobacterial whole cell or extract have been designed and tested in clinical trials in South Africa, Zambia, Kenya, Tanzania. For instance, M72 vaccine comprising M.tb fusion protein MTB32A+MTB39A with AS01E adjuvant has entered Phase IIb trial and has shown 54% of efficacy against TB disease (Van Der Mereen, Hatherill et al, NEJM 2018).
Other vaccines such as H56 prevention of recurrence that aims to prevent from TB recurrence has entered Phase II trial in South and Tanzania in the tests of Dr Elisa Nemes and others.
H4:IC31, another candidate subunit vaccine that entered Phase II trial showed 30.5% efficacy against sustained QFT conversion. Although modest, this efficacy supports that subunit vaccines may be promising in the clinical development of new vaccine candidates against TB (Nemes, Geldenhuys, Rozot et al. NEJM 2018). Many challenges have to be revealed to validate next generation of TB vaccines. These challenges comprise models to understand vaccine design and pathogenesis, experimental medicine and human immune correlates of protection.
Article by Abdouramane Camara, IBMC, Strasbourg-France