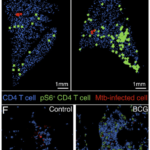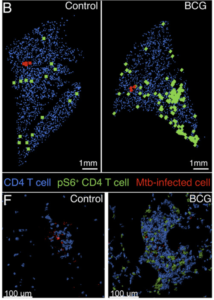
Quantitative histocytometry was used to identify the location of CD4 T cells (blue) and p-S6+CD4 T cells (green) relative to infected cells (red) in lung sections at day 10 (B) and sites of infection at day 14 (F). Source: Delahaye et al., 2019 Journal of Immunology
Murine models have shown that Bacille Calmette-Guérin (BCG) vaccination does not alter early innate immune responses against tuberculosis (TB). This finding was predominantly attributed to the inability of BCG to induce long-lasting lung resident T cells and improve T cell recruitment after M.tb infection. However, other studies have shown that BCG vaccination does induce CD4+ lung resident T cells, suggesting that delayed T cell recruitment is not the sole reason for reduced BCG protection.
Early M.tb infection kinetics in mice have shown that M.tb first infects alveolar macrophages (AMΦ), then disseminates to neutrophils and monocyte-derived MΦ (MDMΦ). To determine if BCG affects early kinetics of M.tb infection and lymphocyte recruitment, Delahaye et al., compared immune responses between naive- and BCG-vaccinated mice infected with M.tb**. They showed that though BCG vaccination did not reduce bacterial burden during the first 2 weeks post infection, they did observe differences M.tb infection patterns of myeloid cells. Specifically BCG vaccination reduced M.tb burden in AMΦ but enhanced MDMΦ recruitment to the lung, as well as M.tb dissemination to neutrophils and MDMΦ. Delahaye et al., also showed that BCG induced lung resident CD4-T cells contribute to pulmonary recruitment of MDMΦ and promotes M.tb infection of other myeloid populations. These findings elucidate novel mechanisms by which BCG alters early innate M.tb immune responses, as well as highlight an area of improvement for future vaccine design.
**M.tb infection: direct lung inoculation with low dose 50-100CFU of H37rv M.tb
Journal article: Delahaye et al., 2019. Cutting Edge: Bacillus Calmette–Guérin–Induced T Cells Shape Mycobacterium tuberculosis Infection before Reducing the Bacterial Burden. Journal of Immunology