- Tumours are organ-like structures composed of different cell types whose interactions are required to promote their maintenance, growth and metastasis.
- Increasing evidence indicates that the tumour microenvironment (TME) can dictate aberrant cellular function and play a critical role in the subsequent development of more advanced and refractory malignancies.
- TME is constituted of cancer-associated fibroblast (CAFs), endothelial cells, immune cells like tumour associated macrophages (TAMs), myeloid derived suppressor cells (MDSCs), lymphocytes, adipocytes, cancer stem cells (CSCs), mesenchymal stromal cells (MSCs), cell products as extracellular matrix (ECM) proteins, growth factors and cytokines.
- Most elements work together to promote tumour survival and/or evasion of the immune system. Recent evidence indicates that TME can function by protecting CSCs from chemotherapy.
- Fibroblasts are an abundant heterogeneous population that maintains the structural organisation within the tissues. While normal fibroblasts suppress malignant cells, CAFs can promote carcinogenesis.
- The vascular network is formed through generation of new vessels from existing vessels in a dynamic process called angiogenesis.
- The stromal cells influence the composition of innate and adaptive immune cells into the tumour as well as the permeability of the vasculature.
- Strong evidence supports the role of microenvironmental inflammation in tumourigenesis and the characterisation of the immune content has been used to predict patient outcome (Junttila and de Sauvage 2013).
- Although in homeostasis settings, the stroma play a role in immune surveillance.
- Cancer cells are capable of developing mechanisms to modify their antigenicity and to evade surveillance by converting the neighbourhood into a pathologically irreversible entity (Chen et al. 2015).
- A number of in vitro models, including monolayer cultures, three-dimensional (3D) cultures and 3D organotypic tissue slice cultures, have provided platforms to study the role of different TME, demonstrating active signals that favour cancer survival, immunosuppression and drug resistance. Thus, TME have become a promising therapeutic target.
- Once tumours become a pro-tumoural landscape, stromal cells co-evolve with cancer cells and use their plasticity to respond to oncogenic challenges.
- The studies of TME during remission suggest a TME dynamics induced by therapy, as in patients with complete remission the TME became normal.
- A frequent observation by pathologists suggests that while some malignancies resemble morphologically and biochemically the organ of origin, many others are less differentiated and even embryonic-like (Bissell and Labarge 2005).
- Metastasis is associated with 90% of mortality among solid tumour patients.
- The mechanisms of migration of cancer cells from original tumour comprise local invasion, intravasation, survival in periphery, extravasation and re-colonisation. TME can promote the initial dissemination creating a permissive niche at distant organ, the formation of a receptive landscape before the arrival of tumour cells enhances metastasis efficiency, substantiating the “seed and soil” hypothesis postuled by Paget (Paget 1989). Recent evidences show a new mechanism to prepare the metastatic niche that is dependent on exosomal secretion (Hoshino et al. 2015).
- Even the heterogeneity of cell populations in the TME, a majority produce pro-inflammatory elements, like tumour necrosis factor (TNF), interleukin-6 (IL-6), interleukin-1b (IL-1b), monocyte chemotactic protein 1 (CCL2), stroma derived factor 1 (SDF-1/CXCL12), interleukin-8 (IL-8), Transformant growth factor b (TGFb). Moreover, the activation of the NF-kB, AP-1 and STAT-3 pathways support a dominant pro-inflammatory condition. MSCs can differentiate in CAFs under concentrations of VEFG, CXCL8, MCP1, CCL2, CXCL12, BMPs, IL-1, TNF and PDGF.
- The modulation of microenvironment in bone marrow (BM) during hematological diseases is even more complex. The bone marrow (BM) microenvironment regulates the properties of healthy hematopoietic stem cells (HSCs) localised in specific niches. In mice, three distinct microenvironmental niches have been identified in the BM, the osteoblastic (endosteal), vascular and reticular niches. These niches provide sanctuaries where subsets of leukemic cells escape chemotherapy-induced death and acquire a drug-resistant phenotype where leukemia cells are able to remodel the BM niches into malignant niches, which better support neoplastic cell survival and proliferation by hijacking the HSCs niches.
- The key roles played by the BM microenvironment in sustaining ALL is documented by the seminal observation that in ALL patients, a higher lymphoblast recovery after culturing leukemic cells with BM-derived stromal cells, was associated with a poorer outcome.
- Targeting the TME has become interesting. The inhibition of enzymes such as cysteine proteases, cathepsins and heparanase, offers the possibility to block multiple processes in the TME as well as the disruption of communication in the malignant niche like CXCR4-CXCL12 axis or even targeting inflammation.
Role of Endothelial cells in tumour microenvironmental inflammation
- During an acute or chronic inflammatory response, leukocytes usually exit the circulation and enter surrounding tissue via post-capillary venules.
- The endothelial cells that line the lumen of these vessels are active participants in this process.
- The endothelial cell layer is one cell thick, and tight junctions exist between cells, that normally prevent the leakage of fluid into surrounding tissues.
- Following activation, endothelial cells undergo structural and functional changes that allow leakage of fluid and plasma proteins into the surrounding tissue.
- In addition, they upregulate cytokines and adhesion molecules, important for the tethering and tight adhesion of leukocytes.
Cellular and Receptor: Ligand events in inflammation
- The initial contact between leukocytes and the vascular endothelium is mediated by selectins (L-,E-, and P) and their glycosylated ligands leading to loose and transient adherence of inflammatory cells (rolling) to the vascular endothelium (Figure 1).
- Subsequently, Integrins and their Ig superfamily ligands mediate tighter adhesion, locomotion and transendothelial migration.
- Inflammation is characterised by the capture of leukocytes by loose bonding of the leukocyte to the endothelial cell.
- Because the bond between selectins and their carbohydrate ligands is transient and of low affinity, the leukocytes are actually rolled over the vessel wall by the force of blood flow passing over.
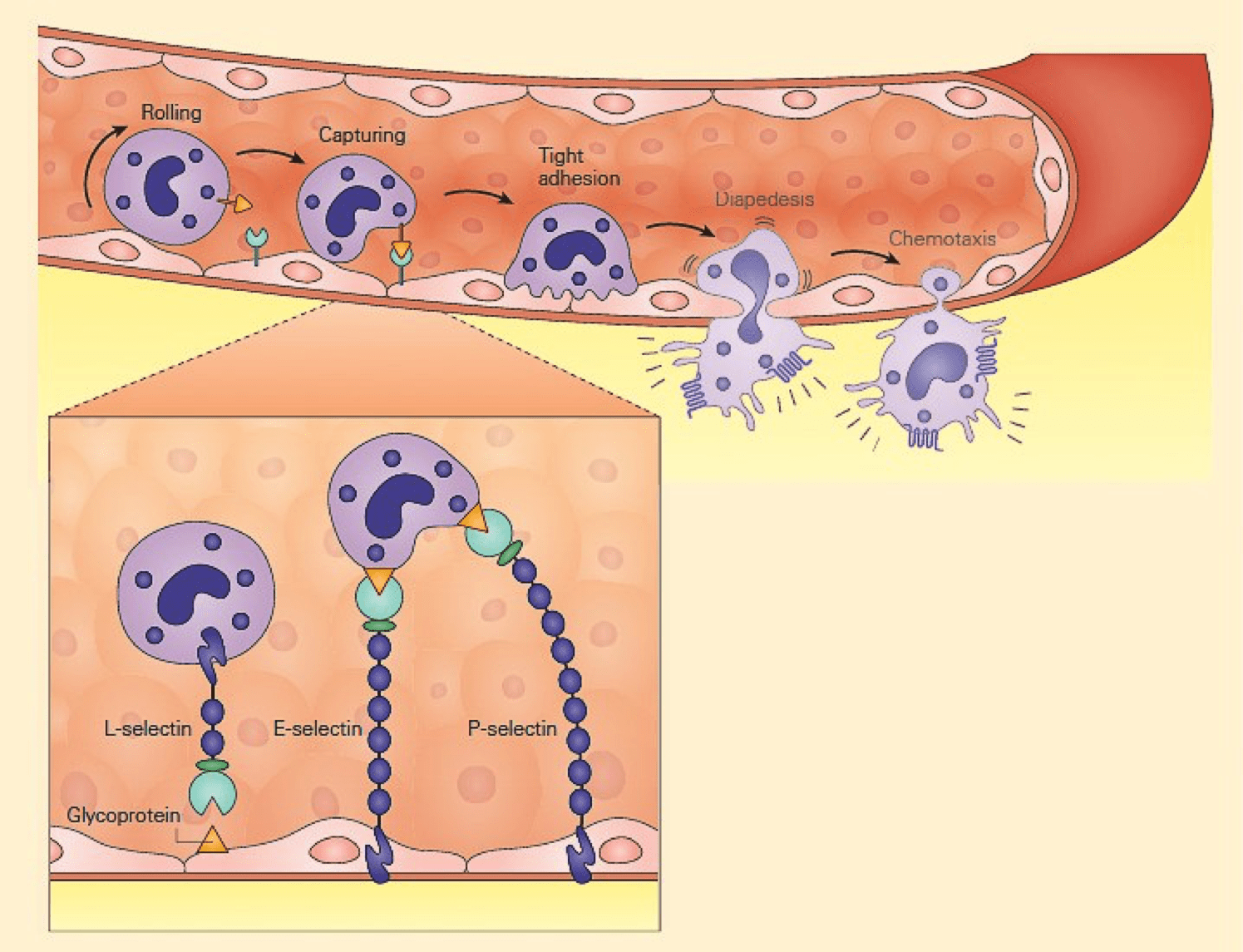
Figure 1: Schematic representation of the capture phase of the leukocyte-endothelial interaction showing the loose binding of selectins to glycoproteins. [Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
- Another set of adhesion molecules that participate in the leukocyte-endothelial interaction involved in inflammation is the immunoglobulin superfamily of molecules.
- The predominant molecules of this family that play a role in leukocyte endothelial adhesion are intercellular adhesion molecules1 and 2 (ICAM-1, and ICAM-2), and vascular cell adhesion molecule 1 (VCAM-1) – shown in Figure 2.
- Variable numbers of these molecules are expressed on the surface both constitutively and after cellular stimulation.
- For these molecules to function properly and bind to integrin ligands, integrins first need to become activated; this occurs when the cell that bears the integrin is stimulated sufficiently by one of the proinflammatory mediators or chemoattractants described previously.
- When activated, the integrin molecule can form a high affinity bond with its counter-ligand, the strength of which causes the leukocyte to stop rolling and adhere tightly.
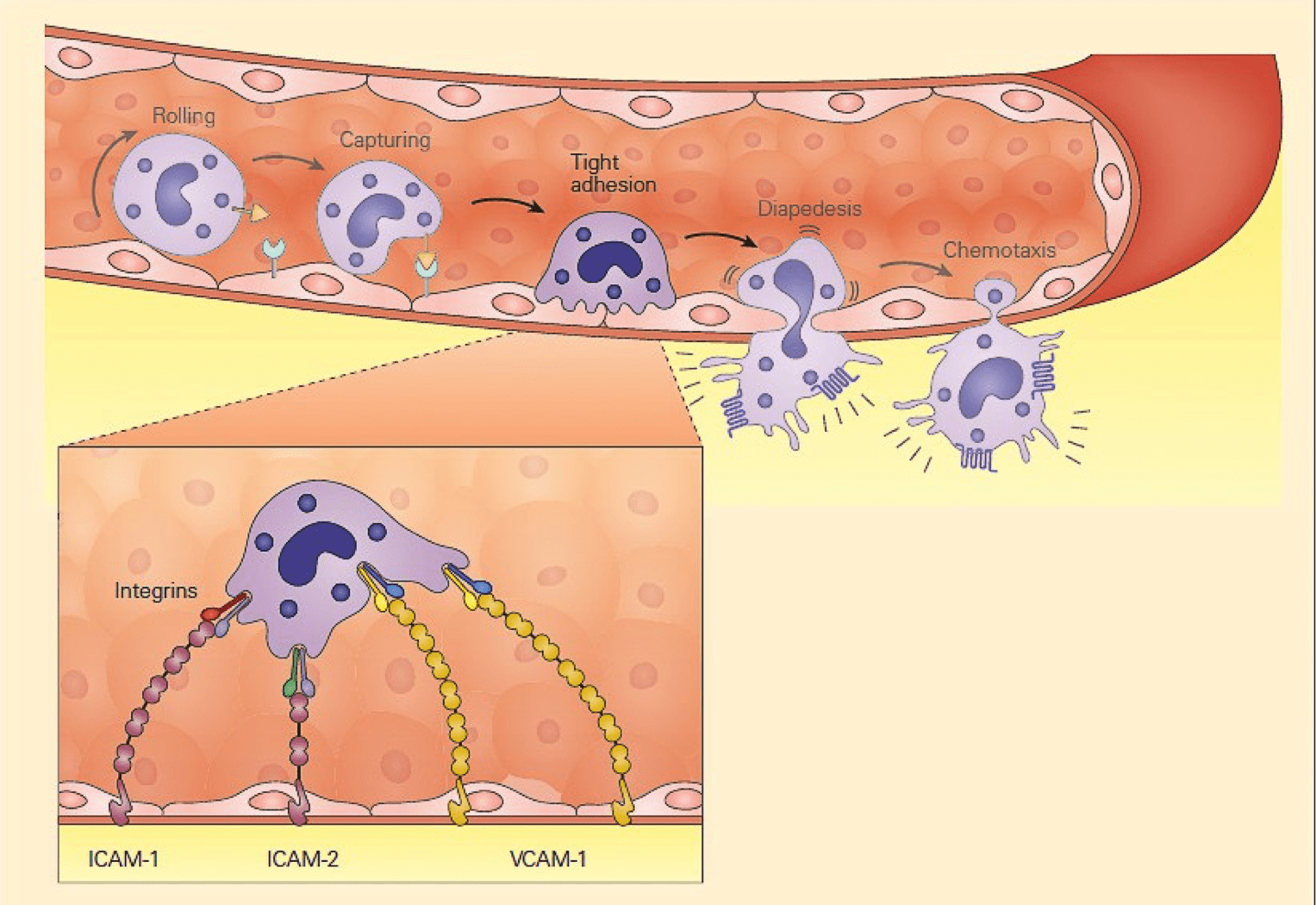
Figure 2: Schematic representation of the tight adhesion phase of the leukocyte-endothelial cell interaction showing the firm binding brought about by the interaction of integrins on the leukocyte membrane with ICAMs on the endothelium. [Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
Role of Selectins in Inflammation
- During inflammation, E-selectin plays an important part in recruiting leukocytes to the site of injury.
- The local release of cytokines IL-1and TNF by damaged cells induces the overexpression of E-selectin on endothelial cells of nearby blood vessels.
- Leukocytesin the blood expressing the correct ligand will bind with low affinity to E-selectin, causing the leukocytes to “roll” along the internal surface of the blood vessel as temporary interactions are made and broken.
- As the inflammatory response progresses, chemokines released by injured tissue enter the blood vessels and activate the rolling leukocytes, which are now able to tightly bind to the endothelial surface and begin making their way into the tissue.
- P-selectin has a similar function, but is expressed on the endothelial cell surface within minutes as it is stored within the cell rather than produced on demand.
- L-selectin is expressed on neutrophils, a subset of T cells, and monocytes.
- Unlike either P-selectin and E-selectin, L-selectin is constitutively expressed.
- In addition, neutrophils shed L-selectin in response to chemotactic stimuli (including IL-8, C5a, and the surrogate bacterial chemoattractant N-formyl-methionyl-leucyl-phenylalanine), but not in response to immune complexes.
- L-selectin binds to CD34 and other glycosylated proteins on the surface of vascular endothelial cells.
Table 1: Various integrins, selectins, and members of the immunoglobulin superfamily of proteins are involved in the leukocyte endothelial interactions of inflammation.
| Adhesion molecule | Specific cellular location | Ligand(s) | Primary function |
|---|---|---|---|
| 1. Selectins (transient interaction): | |||
| P-selectin | Platelet and endothelial cell surface expression after stimulation | Glycoproteins | Mediate cellular margination and rolling |
| E-selectin | Exclusively expressed on stimulated endothelial cells | Glycoproteins | |
| L-selectin | Constitutively expressed on neutrophils, monocytes, and a T cell subset Shed from neutrophils after stimulation with chemoattractants |
Glycoproteins | |
| 2. Immunoglobulinsuperfamily (strong interaction): | |||
| ICAM-1 (CD54) | Low-level constitutive endothelial cell expression Increased endothelial cell expression with stimulation |
LFA-1, Mac-1 | Play a major role in leukocyte-endothelial adhesion |
| ICAM-2 (CD102) | High-level constitutive endothelial cell expression No increase with stimulation |
LFA-1, Mac-1 | |
| ICAM-3 (CD50) | Lymphocytes | LFA-1 | |
| ICAM-4 (CD242) | Erythrocytes | LFA-1, Mac-1 | |
| VCAM-1 (CD106) | No constitutive endothelial cell expression Increased endothelial cell expression with stimulation |
VLA-4 | |
| 3. Integrins (strong interaction) | |||
| LFA-1 (CD11a/CD18b) | Lymphocytes | ICAM-1, ICAM-2, ICAM-3, ICAM-4 | Leukocyte migration, phagocytosis, and growth and development |
| Mac-1 (CR3, CD11b/CD18b) | Neutrophils and monocytes | ICAM-1, ICAM-2 | |
| gp 150/95 (CD11c/CD18b) | Neutrophils and monocytes | ICAM-1, ICAM-2 | |
| VLA-4 (very late antigen-4) | Lymphocytes, monocytes, and eosinophils | VCAM-1 |
Diapedesis and Chemotaxis of Leukocytes Toward an Inflammatory Stimulus
- Following tight adhesion, leukocytes migrate for a short distance along the endothelial surface until they arrive at a junction between endothelial cells.
- The leukocytes then squeeze between endothelial cells to gain access to surrounding tissue.
- This process, called diapedesis, is thought to be mediated by adhesion molecules named platelet/endothelial celladhesion molecule-1 (PECAM-1) and CD99.
- This movement of cells is part of the overall process of chemotaxis in which the movement of cells is directed by chemoattractants such as products of bacterial invasion or the complement components C3a and C5a.
Quiz
References and Links
- Bissell, Mina J, and Mark A Labarge. 2005. “Context, Tissue Plasticity, and Cancer: Are Tumor Stem Cells Also Regulated by the Microenvironment?” Cancer Cell 7 (1): 17–23.
- Chen, Fei, Xueqian Zhuang, Liangyu Lin, Pengfei Yu, Ying Wang, Yufang Shi, Guohong Hu, et al. 2015. “New Horizons in Tumor Microenvironment Biology: Challenges and Opportunities.” BMC Medicine 13 (1). BioMed Central: 45.
- Hoshino, Ayuko, Bruno Costa-Silva, Tang-Long Shen, Goncalo Rodrigues, Ayako Hashimoto, Milica Tesic Mark, Henrik Molina, et al. 2015. “Tumour Exosome Integrins Determine Organotropic Metastasis.” Nature 527 (7578). Nature Publishing Group: 329–35.
- Junttila, Melissa R., and Frederic J. de Sauvage. 2013. “Influence of Tumour Micro-Environment Heterogeneity on Therapeutic Response.” Nature 501 (7467). Nature Publishing Group: 346–54.
- Paget, S. 1989. “The Distribution of Secondary Growths in Cancer of the Breast. 1889.” Cancer Metastasis Reviews 8 (2): 98–101.
- Mauge, L. et al. Control of the Adaptive Immune Response by Tumor Vasculature. Frontiers in Oncology. (4): 61. (2014)
- Ye, W. The Complexity of Translating Anti-angiogenesis Therapy from Basic Science to the Clinic. Developmental Cell. (37): 114–125. (2016)
- Albini, A. et al. Cancer stem cells and the tumor microenvironment: interplay in tumor heterogeneity. Connect Tissue Res. 56(5): 414–425. (2015)
- Balkwill, F., Capasso, M. & Hagemann, T. The tumor microenvironment at a glance. Journal of Cell Science 125, 5591–5596. (2012)
- Flores-Figueroa, E. & Gratzinger, D. Beyond the Niche: Myelodysplastic Syndrome Topobiology in the Laboratory and in the Clinic. Int. J. Mol. Sci. (17): 553. (2016)
- Joyce, J. & Fearon, D. T cell exclusion, immune privilege, and the tumor microenvironment. Science. (348): 74-80. (2015)
- Schepers, K., Campbell, T. & Passegué, E. Normal and Leukemic Stem Cell Niches: Insights and Therapeutic Opportunities. Cell Stem Cell. 16(3): 254–267. (2015)