- Patient Presentation
- History
- Differential diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final outcome
- References
- Evaluation – Questions & answers
Patient Presentation
A 2 year old boy presented to a district hospital with decreased oral intake, listlessness and fever. On arrival he was adequately resuscitated but continued to have spiking fevers and a depressed level of consciousness.
Acknowledgement
This case study was kindly provided by Barclay Stewart, Medical University of South Carolina, Fogarty International Clinical Research Scholar, Nairobi, Kenya
History
Six months ago the patient presented to the hospital with a two day history of irritability, decreased appetite, discomfort on lying down, recurrent fever, profuse sweating and diarrhea, no vomiting. On admission he was lethargic and dehydrated which worsened over a few hours and culminated in a seizure. He had no prior history of seizures. He was then diagnosed with severe malaria. He was treated appropriately and discharged 2 weeks later with no residual effects.
Past medical and surgical history
- There is no additional significant medical or surgical history.
- Road to health card shows all growth parameters to be within normal limits, with all vaccinations up to date.
Family and social history
- He lives with his mother, father, and two older siblings who are all healthy.
- His mother was recently tested and is HIV negative; his father has not been tested.
- Their home, which has electricity and water, is located in a low-lying area near Musina, a town in South Africa’s Limpopo province. This is the country’s most northerly located town, with a seasonal high rate of malaria transmission from October through May.
Travel History
No travel outside of Musina since birth.
Differential diagnosis
- Encephalitis
- Meningitis
- Gastroenteritis with severe dehydration
- Pneumonia
- Toxic Shock Syndrome
- Typhoid Fever
- Brucellosis
- Relapsing Fever
- Katayama Fever
- Urinary tract infection
- Bacteraemia
Examination
On appearance the child is miserable and toxic looking.
Vitals
- Pulse – 166
- Respiratory Rate – 34
- Temperature – 39.8
- Pulse-Oxygen – 95%
Height and weight were in the 65 percentile
General
- Eyes were sunken and jaundiced.
- No lymphadenopathy
ENT
- Erythematous, non bulging tympanic membranes.
- Non-inflamed nasal passage, no discharge.
- Pale oral mucosa
Eyes
- No papilloedema
- No retinal heamorrhages
Chest
- Midline trachea
- Chest shape normal in appearance, tachypnoea present
- Mild subcostal retractions.
- Clear on auscultation bilaterally.
Cardiovascular
- Tachycardia with a regular rhythm.
- Normal S1 and S2 with a 2/6 mid systolic murmur best auscultated over the upper left sternal border with minimal radiation.
- Bounding pulses felt radially, femorally and dorsalis pedis
- Capillary refill within 2 seconds.
Abdomen
- Normal on inspection.
- Bowel sounds diminished but present.
- No hepatomegaly.
- 4cm splenomegaly.
Neurological
- Child listless though attempts to follow commands.
- Not resisting or crying in response to aggravating stimuli.
Investigations
| Examination | Value | Normal Limits |
|---|---|---|
| WBC | 12 | (4 – 12 x 109/l) |
| HB | 7.2 | (12.1 – 15.2 g/l) |
| HCT | 0.22 | (.31 – .42 g/l) |
| Platelets | 222 | (140 – 450 x 109/l) |
| LDH | 294 | (70 – 250 U/l) |
| Haptoglobin (free serum) | 23 | (27-139 mg/dl) |
| Reticulocyte Count | 6 | (0.8 – 4% RBC) |
| Metabolic Panel | ||
| AST | 52 | (5 – 35 U/l) |
| ALT | 101 | (25 – 120 U/l) |
| Total Bilirubin | 2.1 | (0.1 – 1.3 mg/dl) |
| Direct Bilirubin | 0.1 | (0.0 – 0.3 mg/dl) |
| Sodium | 143 | (135 – 147 mmol/l) |
| Potassium | 4.0999999999999996 | (3.5 – 5.1 mmol/l) |
| Bicarbonate | 25 | (22 – 33 mmol/l) |
| Chloride | 93 | (95 – 107 mmol/l) |
| Anion Gap | 25 | (8 – 16 meg/l |
| Creatinine (serum | 1.8 | (0.7 – 1.3 mg/l) |
| BUN (serum) | 21 | (7 – 20 mg/dl) |
| Glucose (serum) | 50 | (65 -110 mg/dl) |
| Urine Analysis | ||
| Spec. Gravity | 1.0289999999999999 | (1.010 – 1.030) |
| pH | 6.3 | (4.8 – 7.5) |
| Ketones | Trace | Absent |
| Protein | Trace | Absent |
| Bilirubin | Trace | Absent |
| Glucose | Absent | Absent |
| RBC | Trace | Absent |
| WBC | Trace | Absent |
| Blood Culture | Negative | |
| Urine Culture | Negative | |
| Malaria Thick and Thin Smear | Positive for P. falciparum | |
| CSF Chemistries | Normal | |
| CSF Culture | Negative |
Discussion
 Epidemiology
Epidemiology
Malaria causes between 300 and 500 million clinical episodes worldwide and 0.5 to 3 million deaths annually, disproportionately affecting immune naïve children under the age of 5 years living in sub-Saharan Africa.
Human malaria infection is caused by four protozoa species of the genus Plasmodium. These are P.falciparum, P. malariae, P. vivax, and P. ovalae, of which the preponderance of severe malaria and mortality is due to P.falciparum. Children living in endemic areas typically have a primary malaria episode during their first few years of life and most toddlers and juveniles develop some degree of acquired immunity against severe disease but still experience periodic clinical episodes. Those who survive to adulthood are often clinically immune, however, low grade parasitaemia is often present but causes few symptoms. Adults in endemic areas maintain low-grade infections throughout the transmission season. Endemicity is typically defined as parasitaemia rates or palpable spleens in children aged 2-9 years. The categories include holoendemic where the rate is >75% (transmission of infection is year round and the bulk of mortality is seen in infants), hyperendemic where the rate is 51-75% (mortality is also mostly seen in infants), mesoendemic where the rate is 11-50% (regular seasonal transmission affecting infants, toddlers and adults who develop chronic ill health) and hypoendemic which is <10% (occasional epidemics, whole population is susceptible to severe and fatal disease). Clinical immunity also fails if a person moves away from an endemic area and during pregnancy.
P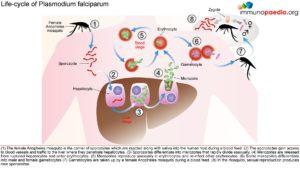 arasitology – Malaria Life-Cycle
arasitology – Malaria Life-Cycle
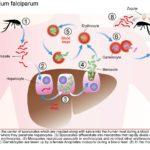
The female Anopheles mosquito inoculates the host with 10 to 100 malaria sporozoites from her salivary glands during a blood meal. These microscopic motile forms of the malaria parasite are carried via the bloodstream to the liver. Within 30 minutes, those sporozoites not bound by previously formed antibodies, invade and begin replicating in hepatocytes. Parasites not destroyed by cytotoxic T lymphocytes in the liver replicate for 2-10 days creating merozoites. Tens of thousands of merozoites are released into the bloodstream as the hepatocyte bursts. Each merozoite is then able to bind, invade, and infect erythrocytes. After red blood cell (RBC) infection, each merozoite matures to form a highly metabolically active trophozoite, which replicates asexually to become multinucleate schizonts. As the schizonts enlarge they rupture erythrocytes 48 hours after their formation which results in 20-30 new merozoites which continue the cycle. Some sexual forms of the parasite develop during this erythrocytic stage; these gametocytes are responsible for infecting the salivary glands of female Anopheles mosquitoes. The gametes mature into ookinetes then into an oocyst. The oocyst ruptures and releases sporozoites which can then infect another host during a blood meal.
 Pathogenesis and Clinical Features
Pathogenesis and Clinical Features
A person’s first infection usually creates no symptoms for 7-10 days, which is followed first by nonspecific symptoms such as headache, fatigue, abdominal discomfort and muscle aches. This is then followed by fever. During this latent period, parasite maturation occurs in the liver and parasites undergo a cycle of blood stage replication. Symptoms begin when the parasites undergoing an asexual blood cycle, reach threshold density sufficient to initiate the host’s pathogenic immune response process. Fever, malaria’s hallmark, is due to parasite-derived molecules released from ruptured host cells. These molecules activate host inflammatory cells, such as macrophages, which secrete pro-inflammatory pyrogenic cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)–α. As parasites synchronise their replication cycles the fever becomes periodic. Although childhood febrile convulsions can occur, generalised seizures are typically associated with P.falciparum infections and may herald cerebral malaria. Splenomegaly results from massive reticuloendothelial system activation to clear parasitised erythrocytes. Mild hepatomegally is common in young children, while mild jaundice is more common in adults. Anaemia is also common and is partly due to the phasic rupture of RBCs by mature schizonts, splenic sequestration of red blood cells and ineffective erythropoiesis.
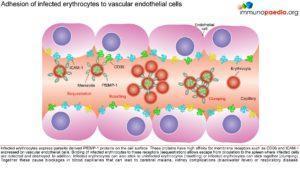
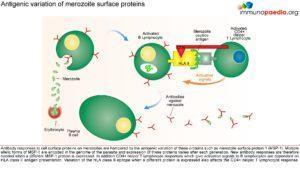 Parasitised erythrocytes circulate to the microvasculature where they bind to the scavenger receptor CD36 or the intercellular adhesion 1 (ICAM-1) and occlude microvessels. Binding of infected erythrocytes to these receptors (sequestration) allows the cells to escape from circulation to the spleen where infected cells are detected and destroyed. In addition, infected erythrocytes can also stick to uninfected erythrocytes (rosetting) or infected erythrocytes can stick together (clumping). In particular, parasitized RBCs adhere to the endothelial lining of capillaries in the brain, kidneys, gut and liver. Inflammation caused by these parasite-receptor interactions and release of immune stimulatory molecules from infected RBCs upon rupture are thought to be responsible for organ specific pathology such as cerebral malaria, renal disease, Blackwater Fever (due to widespread intravascular haemolysis giving rise to dark urine) and respiratory distress
Parasitised erythrocytes circulate to the microvasculature where they bind to the scavenger receptor CD36 or the intercellular adhesion 1 (ICAM-1) and occlude microvessels. Binding of infected erythrocytes to these receptors (sequestration) allows the cells to escape from circulation to the spleen where infected cells are detected and destroyed. In addition, infected erythrocytes can also stick to uninfected erythrocytes (rosetting) or infected erythrocytes can stick together (clumping). In particular, parasitized RBCs adhere to the endothelial lining of capillaries in the brain, kidneys, gut and liver. Inflammation caused by these parasite-receptor interactions and release of immune stimulatory molecules from infected RBCs upon rupture are thought to be responsible for organ specific pathology such as cerebral malaria, renal disease, Blackwater Fever (due to widespread intravascular haemolysis giving rise to dark urine) and respiratory distress
Clinical Fea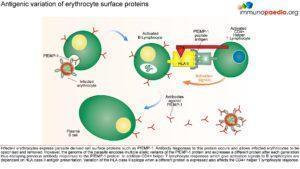 tures of Severe Falciparum Malaria
tures of Severe Falciparum Malaria
Correctly treated uncomplicated P.falciparum carries a mortality rate of 0.1%. When organ dysfunction or parasitaemia of >3% occurs mortality also increases dramatically. These manifestations, which were also seen in this clinical case, include:
Cerebral Malaria
Onset may be gradual or sudden following a convulsion. Features include obtundation, delirium, abnormal behaviour and coma. Focal neurologic signs and meningism do not typically occur. Fifteen percent of children who survive cerebral malaria, especially when associated with hypoglycaemia, coma and anaemia will have some residual neurologic deficit.
Hypoglycaemia
Common complication that is associated with a poor prognosis, particularly in children and pregnant women. Hypoglycaemia is due to a failure of hepatic gluconeogenesis and an increase in glucose consumption by host and parasite. This may manifest as an added complication during treatment as Quinine is also a potent stimulator of insulin secretion.
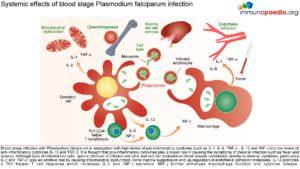 Lactic Acidosis
Lactic Acidosis
Coexists with hypoglycaemia and contributes to death. Caused by anaerobic glycolysis in tissues where parasites interfere with microcirculation, cytokine induced mitochondrial dysfunction, hypovolaemia, lactate production by parasites and poor hepatic and renal clearance.
Haematologic Pathology
Anaemia due to increased destruction and removal or red blood cells and dyserythropoesis.
Mild thrombocytopaenia
Mild coagulation abnormalities
Bleeding and DIC in more severe cases
Renal pathology
Interference in microcirculation resulting in tubular necrosis and acute renal failure, more common in adults.
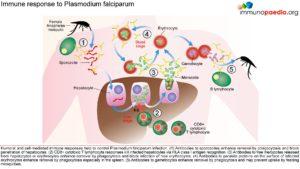
Host Response-Immunology
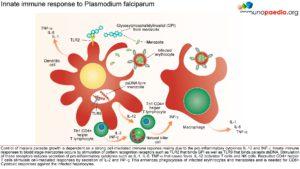 The innate immune responses have already been discussed above. Regarding acquired immune responses many studies show that antibodies and T cells are vital to the development of a protective immune response against malaria infection. Specific immune responses are required to defuse the infection at multiple points in the parasite life cycle.
The innate immune responses have already been discussed above. Regarding acquired immune responses many studies show that antibodies and T cells are vital to the development of a protective immune response against malaria infection. Specific immune responses are required to defuse the infection at multiple points in the parasite life cycle.
- Antibody responses are induced during the sporozoite stage. Antibody bound sporozoites are prevented from invading hepatocytes.
- CD8+ T cells have been shown to be cytotoxic against maturing sporozoite infected liver cells.
- Both of these responses are potentially able to terminate the infection before the onset of clinical disease caused by the release of merozoites from hepatocytes and subsequent RBC invasion and rupture.
- CD4+ T cells are a requisite for the production of merozoite neutralising antibodies by B cells and the activation of macrophages which secrete interferon (INF) –γ to enhance parasitized RBC.
- The host is also able to develop transmission-blocking antibodies directed to gametocyte specific antigens. These antibodies hinder the development of the parasite within the mosquito vector, thereby preventing further infections. Though this immune response is not particularly valuable to the infected host, it does assist in reducing population level transmission.
M alaria has evolved unique strategies to evade the host immune system and is therefore able to cause repeated and serious infections. To overcome and control this disease one important aspe
alaria has evolved unique strategies to evade the host immune system and is therefore able to cause repeated and serious infections. To overcome and control this disease one important aspe
ct is to develop an efficacious and safe vaccine. In order to do this it is necessary to understand the host immune responses and the immune evasion techniques that malaria employs.
Download images for this case
Treatment
It is recommended that patients receive prompt and effective treatment. Ideally, treatment should be initiated in a hospital setting. The choice of chemotherapy for malaria is dependent on the severity of disease, the known or suspected resistance pattern of the parasite in the area where the malaria infection was acquired, the species of parasite, patient characteristics (age, pregnancy, co-morbidity, allergies, other medications) and the presence or absence of vomiting. In South Africa, malaria treatment varies in the different provinces due to differences in the resistance patterns. These treatment guidelines may not be appropriate for infections contracted in other countries with high levels of multi-drug resistance.
Download images for this case
Final outcome
The patient was treated with IV artesunate and anti-pyretics for 3 days. IV antibiotics were started on admission as there was no confirmatory diagnosis at the time and culture results were not yet available. On the third day the child was markedly improved. He was started on a full course of mefloquine on receiving laboratory results which confirmed infection with P.falciparum. Upon discharge there were no neurologic sequelae. He and his family were counseled on the use of insecticide-treated bed nets and indoor residual spraying.
Download images for this case
References
Guerin, P.J., et al. (2002). Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect Dis. 2(9): p. 564-73.
Link to Abstract
Ferreira, M.U et al. (2004). Antigenic diversity and immune evasion by malaria parasites. Clin Diagn Lab Immunol. 11(6): p. 987-95.
Link to Abstract
May, J. et al. (1999). High rate of mixed and subpatent malarial infections in southwest Nigeria. Am J Trop Med Hyg. 61(2): p. 339-43.
Link to Abstract
Rosenberg, R. et al. (1990). An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans R Soc Trop Med Hyg. 84(2): p. 209-12.
Link to Abstract
Ponnudurai, T. et al. (1982). Mosquito transmission of cultured Plasmodium falciparum. Trans R Soc Trop Med Hyg. 76(2): p. 278-9.
Link to Abstract
Ferreira, M.U et al. (1998). The IgG-subclass distribution of naturally acquired antibodies to Plasmodium falciparum, in relation to malaria exposure and severity. Ann Trop Med Parasitol. 92(3): p. 245-56.
Link to Abstract
Nardin, E.H et al. (1993). T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu Rev Immunol. 11: p. 687-727.
Link to Abstract
Nardin, E.H. et al. (1982). Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 156(1): p. 20-30.
Link to Abstract
Inselburg, J. (1983). Gametocyte formation by the progeny of single Plasmodium falciparum schizonts. J Parasitol. 69(3): p. 584-91.
Link to Abstract
Aitman, T.J. et al. (2000). Malaria susceptibility and CD36 mutation. Nature. 405(6790): p. 1015-6.
Link to Abstract
Jenkins, N. et al. (2007). Plasmodium falciparum intercellular adhesion molecule-1-based cytoadherence-related signaling in human endothelial cells. J Infect Dis. 196(2): p. 321-7.
Link to Abstract
McCormick, C.J. et al. (1997). Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J Clin Invest. 100(10): p. 2521-9.
Miller, L.H. et al. (2002). The pathogenic basis of malaria. Nature. 415(6872): p. 673-9.
Abdel-Latif, M.S. et al. (2003). Antibodies to Plasmodium falciparum rifin proteins are associated with rapid parasite clearance and asymptomatic infections. Infect Immun. 71(11): p. 6229-33.
Good, M.F. et al. (1998). Pathways and strategies for developing a malaria blood-stage vaccine. Annu Rev Immunol. 16: p. 57-87.
Hoffman, S.L. et al. (1998). Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science. 244(4908): p. 1078-81.
Link to Abstract
Snewin, V.A et al. (1995). Transmission blocking immunity in Plasmodium vivax malaria: antibodies raised against a peptide block parasite development in the mosquito vector. J Exp Med. 181(1): p. 357-62.
Link to Abstract
Hisaeda, H. et al. (2005). Malaria: immune evasion by parasites. Int J Biochem Cell Biol. 37(4): p. 700-6.
Link to Abstract
Qari, S.H. et al. (1998). Predicted and observed alleles of Plasmodium falciparum merozoite surface protein-1 (MSP-1), a potential malaria vaccine antigen. Mol Biochem Parasitol. 92(2): p. 241-52.
Link to Abstract
Burns, J.M. et al. (1989). A protective monoclonal antibody recognizes a variant-specific epitope in the precursor of the major merozoite surface antigen of the rodent malarial parasite Plasmodium yoelii. J Immunol. 142(8): p. 2835-40.
Link to Abstract
Smith, J.D. et al. (1995). Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 82(1): p. 101-10.
Link to Abstract
Flick, K. et al. (2004). var genes, PfEMP1 and the human host. Mol Biochem Parasitol. 134(1): p. 3-9.
Link to Abstract
Williamson, W.A. et al. (1978). Impairment of the immune response to vaccination after acute malaria. Lancet. 1(8078): p. 1328-9.
Link to Abstract
Takeda, K. et al. (2003). Toll-like receptors. Annu Rev Immunol. 21: p. 335-76.
Link to Abstract
Urban, B.C. et al. (1999). Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 400(6739): p. 73-7.
Link to Abstract
Ocana-Morgner, C. et al. (2003). Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med. 197(2): p. 143-51.
Link to Abstract
Omer, F.M et al. (2003). Differential induction of TGF-beta regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J Immunol. 171(10): p. 5430-6.
Link to Abstract
Shevach, E.M. (2002). CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2(6): p. 389-400.
Hisaeda, H. et al. (2004). Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med. 10(1): p. 29-30.
Download images for this case
Evaluation – Questions & answers
What is the diagnosis?
With regards to parasitized erythrocytes which endothelial receptors do they bind to resulting in occlusion of microvessels?
What are the three ways that infected erythrocytes can bind to occlude microvessels?
Rosetting- infected erythrocytes stick to uninfected erythrocytes
Clumping- infected erythrocytes stick together
What is the benefit of occlusion of microvessels?
Which organs are most affected by occlusion of microvessels?
Describe the immune response required to neutralize malaria parasites at each stage during their development.
Maturing hepatic shizonts – CD8+ T cell
Merozoite – Antibody
Gametocyte – Antibody
Download images for this case